Engineered Male Sterility by Early Anther Ablation Using the Pea Anther-Specific Promoter PsEND1
- PMID: 31293612
- PMCID: PMC6603094
- DOI: 10.3389/fpls.2019.00819
Engineered Male Sterility by Early Anther Ablation Using the Pea Anther-Specific Promoter PsEND1
Abstract
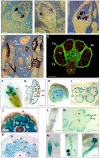
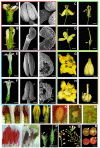
Genetic engineered male sterility has different applications, ranging from hybrid seed production to bioconfinement of transgenes in genetic modified crops. The impact of this technology is currently patent in a wide range of crops, including legumes, which has helped to deal with the challenges of global food security. Production of engineered male sterile plants by expression of a ribonuclease gene under the control of an anther- or pollen-specific promoter has proven to be an efficient way to generate pollen-free elite cultivars. In the last years, we have been studying the genetic control of flower development in legumes and several genes that are specifically expressed in a determinate floral organ were identified. Pisum sativum ENDOTHECIUM 1 (PsEND1) is a pea anther-specific gene displaying very early expression in the anther primordium cells. This expression pattern has been assessed in both model plants and crops (tomato, tobacco, oilseed rape, rice, wheat) using genetic constructs carrying the PsEND1 promoter fused to the uidA reporter gene. This promoter fused to the barnase gene produces full anther ablation at early developmental stages, preventing the production of mature pollen grains in all plant species tested. Additional effects produced by the early anther ablation in the PsEND1::barnase-barstar plants, with interesting biotechnological applications, have also been described, such as redirection of resources to increase vegetative growth, reduction of the need for deadheading to extend the flowering period, or elimination of pollen allergens in ornamental plants (Kalanchoe, Pelargonium). Moreover, early anther ablation in transgenic PsEND1::barnase-barstar tomato plants promotes the developing of the ovaries into parthenocarpic fruits due to the absence of signals generated during the fertilization process and can be considered an efficient tool to promote fruit set and to produce seedless fruits. In legumes, the production of new hybrid cultivars will contribute to enhance yield and productivity by exploiting the hybrid vigor generated. The PsEND1::barnase-barstar construct could be also useful to generate parental lines in hybrid breeding approaches to produce new cultivars in different legume species.
Keywords: Pisum sativum; PsEND1 promoter; barnase; hybrid seeds; male sterility; parthenocarpy; pollen allergens; transgene bioconfinement.
Figures
References
-
- Beltrán J. P., Cañas L. A. (2018). “Grain and forage legumes: nutritional value and agriculture sustainability,” in Functional genomics in Medicago truncatula. Methods and Protocols. Methods in Molecular Biology Series n 1822, eds Cañas L., Beltrán J. P. (New York, NY: Humana Press; ). - PubMed
-
- Beltrán J. P., Roque E., Medina M., Madueño F., Gómez M. D., Cañas L. A. (2007). Androesterilidad inducida mediante ingeniería genética. fundamentos y aplicaciones biotecnológicas. An. R. Acad. Nac. Farm. 73 1237–1264.
-
- Benlloch R., Navarro C., Beltrán J. P., Cañas L. A. (2003). Floral development of the model legume Medicago truncatula: ontogeny studies as a tool to better characterize homeotic mutations. Sex. Plant Reprod. 15 231–241.

