Insertion of [1.1.1]propellane into aromatic disulfides
- PMID: 31293664
- PMCID: PMC6604700
- DOI: 10.3762/bjoc.15.114
Insertion of [1.1.1]propellane into aromatic disulfides
Abstract
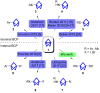
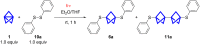
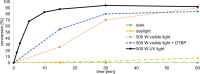
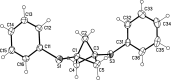
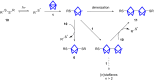
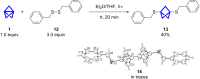
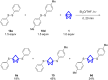
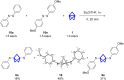
Herein we present the synthesis of symmetrically and unsymmetrically substituted 1,3-bissulfanylbicyclo[1.1.1]pentanes from disulfides and [1.1.1]propellane. Bicyclo[1.1.1]pentanes (BCPs) recently gained interest as rigid linkers and as bioisosters of para-substituted benzene and alkyne moieties. The most promising precursor for BCPs is [1.1.1]propellane (1). The available methods to synthesize BCPs are quite limited and many groups contribute to the development of novel methods. The insertion of 1 into disulfide bonds is known, but has never been thoroughly investigated. In this study, we show that an UV initiated radical reaction can be used to synthesize symmetrically and unsymmetrically substituted BCP sulfides by reaction of [1.1.1]propellane (1) with disulfides. Depending on the ratio of 1 to the disulfide, only the BCP product (with up to 98% yield) or a mixture of BCP and [2]staffane can be obtained. The reaction tolerates functional groups such as halogens, alkyl and methoxy groups. The separation of the corresponding BCP and [2]staffane products is challenging but possible by column chromatography and preparative TLC in most cases. Single crystal X-ray diffraction analysis confirms the rod-like structure of the [2]staffanes that is often required in material applications.
Keywords: [1.1.1]propellane; bicyclo[1.1.1]pentane; bioisosteres; disulfides; linkers.
Figures
References
LinkOut - more resources
Full Text Sources
