Physical energies to the rescue of damaged tissues
- PMID: 31293714
- PMCID: PMC6600852
- DOI: 10.4252/wjsc.v11.i6.297
Physical energies to the rescue of damaged tissues
Abstract
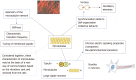
Rhythmic oscillatory patterns sustain cellular dynamics, driving the concerted action of regulatory molecules, microtubules, and molecular motors. We describe cellular microtubules as oscillators capable of synchronization and swarming, generating mechanical and electric patterns that impact biomolecular recognition. We consider the biological relevance of seeing the inside of cells populated by a network of molecules that behave as bioelectronic circuits and chromophores. We discuss the novel perspectives disclosed by mechanobiology, bioelectromagnetism, and photobiomodulation, both in term of fundamental basic science and in light of the biomedical implication of using physical energies to govern (stem) cell fate. We focus on the feasibility of exploiting atomic force microscopy and hyperspectral imaging to detect signatures of nanomotions and electromagnetic radiation (light), respectively, generated by the stem cells across the specification of their multilineage repertoire. The chance is reported of using these signatures and the diffusive features of physical waves to direct specifically the differentiation program of stem cells in situ, where they already are resident in all the tissues of the human body. We discuss how this strategy may pave the way to a regenerative and precision medicine without the needs for (stem) cell or tissue transplantation. We describe a novel paradigm based upon boosting our inherent ability for self-healing.
Keywords: Damaged tissues; Electric fields; Electromagnetic fields; Electromagnetic radiation; Mechanical forces; Photobiomodulation; Physical energies; Stem cells.
Conflict of interest statement
Conflict-of-interest statement: No potential conflicts of interest.
Figures


References
-
- Segura-Valdez ML, Agredano-Moreno LT, Zamora-Cura AL, Lara-Martínez R, Jiménez-García LF. Visualization of internal in situ cell structure by atomic force microscopy. Histochem Cell Biol. 2018;150:521–527. - PubMed
-
- Cosgrove DJ. Nanoscale structure, mechanics and growth of epidermal cell walls. Curr Opin Plant Biol. 2018;46:77–86. - PubMed
-
- Sharma S, LeClaire M, Gimzewski JK. Ascent of atomic force microscopy as a nanoanalytical tool for exosomes and other extracellular vesicles. Nanotechnology. 2018;29:132001. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

