Efficacy of long-term rifaximin treatment for hepatic encephalopathy in the Japanese
- PMID: 31293721
- PMCID: PMC6603506
- DOI: 10.4254/wjh.v11.i6.531
Efficacy of long-term rifaximin treatment for hepatic encephalopathy in the Japanese
Abstract
Background: Hepatic encephalopathy (HE) is a complication of liver cirrhosis and can result in neuropsychological and neuromuscular dysfunctions in patients. Rifaximin, an antibiotic, has been reported to decrease the occurrence of overt HE and also improve cognitive function in studies from Europe and the United States of America. There is not enough evidence of the relationship between the long-term use of rifaximin and its clinical effects in the Japanese.
Aim: To determine the clinical effects of long-term rifaximin therapy in decompensated liver cirrhosis patients, with overt HE or hyperammonemia.
Methods: In this single-center retrospective observational cohort study, we reviewed the data of 38 patients who had taken rifaximin at the dose of 1200 mg/d for more than 24 wk. The primary outcome measured was the efficacy of long-term rifaximin use, and secondary outcome measured was the safety of its long-term use as determined by its influence on portosystemic shunts as well as Escherichia coli-related infections. Moreover, we compared the prognosis between the rifaximin group and control cases, matched for hepatic elasticity assessed by magnetic resonance ela-stography, age, and Child-Pugh classification.
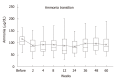
Results: Of the 38 patients included in the study, 12 (31.6%) had overt HE, 27 (71.1%) had complications of esophageal varices, and 9 (23.7%) had hepatocellular carcinoma (HCC). The control group was matched for age, Child-Pugh classification, liver stiffness, and presence of HCC. The median of serum ammonia level before treatment was 104 μg/dL (59-297), and 2 wk after treatment, it significantly decreased to 85 μg/dL (34-153) (P = 0.002). A significantly low value of 80.5 μg/dL (44-150) was maintained 24 wk after treatment. The long-term use of rifaximin did not cause a decline in liver function. Diarrhea occurred in 2 patients, who improved with the administration of probiotics, and there were no cases of aborted rifaximin therapy owing to adverse events. In patients with Child C, the survival was short, but there was no significant difference compared with that of the control group.
Conclusion: Rifaximin therapy improves overt HE. The long-term use of rifaximin in the Japanese is effective and safe.
Keywords: Child-Pugh classification; Hepatic cirrhosis; Hepatic encephalopathy; Magnetic resonance elastography; Rifaximin; Spontaneous portosystemic shunt.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Real-World Setting of Efficacy and Safety of 3 Years of Rifaximin Administration in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study.J Clin Med. 2025 Feb 18;14(4):1358. doi: 10.3390/jcm14041358. J Clin Med. 2025. PMID: 40004887 Free PMC article.
-
Long-Term Efficacy and Safety of Rifaximin in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study.J Clin Med. 2022 Mar 12;11(6):1571. doi: 10.3390/jcm11061571. J Clin Med. 2022. PMID: 35329897 Free PMC article.
-
Changes in the Body Composition and Nutritional Status after Long-term Rifaximin Therapy for Hyperammonemia in Japanese Patients with Hepatic Encephalopathy.Intern Med. 2020;59(20):2465-2469. doi: 10.2169/internalmedicine.5094-20. Epub 2020 Oct 15. Intern Med. 2020. PMID: 33055469 Free PMC article.
-
Long-term management of hepatic encephalopathy with lactulose and/or rifaximin: a review of the evidence.Eur J Gastroenterol Hepatol. 2019 Apr;31(4):434-450. doi: 10.1097/MEG.0000000000001311. Eur J Gastroenterol Hepatol. 2019. PMID: 30444745 Free PMC article. Review.
-
Rifaximin: new therapeutic indication and future directions.Clin Ther. 2011 Jul;33(7):812-27. doi: 10.1016/j.clinthera.2011.06.007. Epub 2011 Jul 7. Clin Ther. 2011. PMID: 21741091 Review.
Cited by
-
Add-on Therapeutic Effects of Rifaximin on Treatment-resistant Hepatic Encephalopathy.Intern Med. 2023 Apr 1;62(7):973-978. doi: 10.2169/internalmedicine.0212-22. Epub 2022 Sep 6. Intern Med. 2023. PMID: 36070941 Free PMC article.
-
Evaluation of the antimicrobial activity of ridinilazole and six comparators against Chinese, Japanese and South Korean strains of Clostridioides difficile.J Antimicrob Chemother. 2021 Mar 12;76(4):967-972. doi: 10.1093/jac/dkaa522. J Antimicrob Chemother. 2021. PMID: 33351917 Free PMC article.
-
The mechanism of dysbiosis in alcoholic liver disease leading to liver cancer.Hepatoma Res. 2020;6:5. doi: 10.20517/2394-5079.2019.29. Epub 2020 Feb 20. Hepatoma Res. 2020. PMID: 32582865 Free PMC article.
-
Real-World Setting of Efficacy and Safety of 3 Years of Rifaximin Administration in Japanese Patients with Hepatic Encephalopathy: A Multicenter Retrospective Study.J Clin Med. 2025 Feb 18;14(4):1358. doi: 10.3390/jcm14041358. J Clin Med. 2025. PMID: 40004887 Free PMC article.
-
Evaluation of the Long-term Administration of Rifaximin for More than Three Years in the Treatment of Repeated and Recurrent Overt Hepatic Encephalopathy.Intern Med. 2021;60(7):1027-1033. doi: 10.2169/internalmedicine.5793-20. Epub 2021 Apr 1. Intern Med. 2021. PMID: 33790139 Free PMC article.
References
-
- Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. - PubMed
-
- Patidar KR, Thacker LR, Wade JB, Sterling RK, Sanyal AJ, Siddiqui MS, Matherly SC, Stravitz RT, Puri P, Luketic VA, Fuchs M, White MB, Noble NA, Unser AB, Gilles H, Heuman DM, Bajaj JS. Covert hepatic encephalopathy is independently associated with poor survival and increased risk of hospitalization. Am J Gastroenterol. 2014;109:1757–1763. - PMC - PubMed
-
- Scarpignato C, Pelosini I. Rifaximin, a poorly absorbed antibiotic: pharmacology and clinical potential. Chemotherapy. 2005;51 Suppl 1:36–66. - PubMed
-
- Phongsamran PV, Kim JW, Cupo Abbott J, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs. 2010;70:1131–1148. - PubMed
LinkOut - more resources
Full Text Sources

