UO2 2+-mediated ring contraction of pyrihexaphyrin: synthesis of a contracted expanded porphyrin-uranyl complex
- PMID: 31293744
- PMCID: PMC6552508
- DOI: 10.1039/c9sc01593k
UO2 2+-mediated ring contraction of pyrihexaphyrin: synthesis of a contracted expanded porphyrin-uranyl complex
Erratum in
-
Correction: UO2 2+-mediated ring contraction of pyrihexaphyrin: synthesis of a contracted expanded porphyrin-uranyl complex.Chem Sci. 2019 Jul 9;10(29):7119. doi: 10.1039/c9sc90138h. eCollection 2019 Aug 7. Chem Sci. 2019. PMID: 31908752 Free PMC article.
Abstract
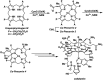
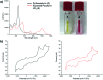
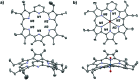
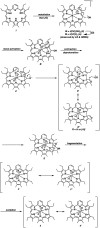
A new mixed hexaphyrin, pyrihexaphyrin (0.1.0.0.1.0) (1), was prepared via an acid catalyzed cyclization between 5,5'-(pyridine-2,6-diyl)bis(pyrrole-2-carbaldehyde) (2) and terpyrrole (3). This expanded porphyrin undergoes a ring contraction upon metallation with uranyl silylamide [UO2[N(SiMe3)2]2] under anaerobic conditions followed by purification over basic aluminum oxide exposed to air. The uranyl-contracted pyrihexaphyrin (0.0.0.0.1.0) complex (4) produced as a result contains a unique structural architecture and possesses a formally 22 π-electron globally aromatic periphery, as inferred from NMR spectroscopy, single crystal X-ray diffraction, and computational analyses. Support for the proposed contraction mechanism came from experimental data and DFT calculations. Proton NMR and mass spectroscopic analysis provided the first insight into expanded porphyrin-mediated activation of the uranyl dication (UO2 2+).
Figures

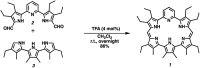
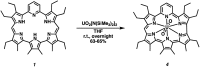
References
-
- Battersby A. R. Nat. Prod. Rep. 2000;17:507. - PubMed
-
- Vannotti A., Porphyrins. Their Biological and Chemical Importance, Hilger and Watts, London, 1955.
-
- Lesage S., Hu H., Durham L. Hydrolog. Sci. J. 1993;38:343.
-
- Shemin D., Russell C. S., Tomio J. M., Grinstein M., Bonkovsky H. L., Guo T. J., Hou W., Li T., Narang T., Thapar M. J. Am. Chem. Soc. Eur. J. Biochem. Compr. Physiol. 1953;1968;2013;7563:4873. 80, 365.
-
- Iida K., Ohtaka K., Kajiwara M., Heldt D., Lawrence A. D., Lindenmeyer M., Deery E., Heathcote P., Rigby S. E., Warren M. J. FEBS J. Biochem. Soc. Trans. 2007;2005;27433:3475. 815.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

