Energy storage: pseudocapacitance in prospect
- PMID: 31293750
- PMCID: PMC6563784
- DOI: 10.1039/c9sc01662g
Energy storage: pseudocapacitance in prospect
Abstract
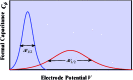
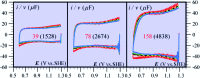
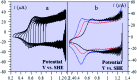
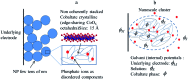
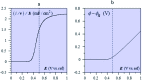
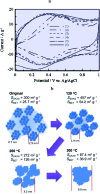
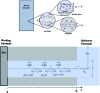
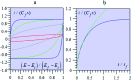
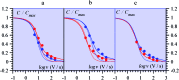
The two main types of charge storage devices - batteries and double layer charging capacitors - can be unambiguously distinguished from one another by the shape and scan rate dependence of their cyclic voltammetric current-potential (CV) responses. This is not the case with "pseudocapacitors" and with the notion of "pseudocapacitance", as originally put forward by Conway et al. After insisting on the necessity of precisely defining "pseudocapacitance" as involving faradaic processes and having, at the same time, a capacitive signature, we discuss the modelling of "pseudocapacitive" responses, revisiting Conway's derivations and analysing critically the other contributions to the subject, leading unmistakably to the conclusion that "pseudocapacitors" are actually true capacitors and that "pseudocapacitance" is a basically incorrect notion. Taking cobalt oxide films as a tutorial example, we describe the way in which a (true) electrical double layer is built upon oxidation of the film in its insulating state up to an ohmic conducting state. The lessons drawn at this occasion are used to re-examine the classical oxides, RuO2, MnO2, TiO2, Nb2O5 and other examples of putative "pseudocapacitive" materials. Addressing the dynamics of charge storage-a key issue in the practice of power of the energy storage device-it is shown that ohmic potential drop in the pores is the governing factor rather than counter-ion diffusion as often asserted, based on incorrect diagnosis by means of scan rate variations in CV studies.
Figures



References
-
- Winter M., Brodd R. J. Chem. Rev. 2004;104:4245–4269. - PubMed
-
- Note that in some cases, the species that cross the interfacial potential drop is an ion rather than an electron. See for example: White H. S., Peterson J. D., Cui Q., Stevenson K. J., J. Phys. Chem. B, 1998, 102 , 2930 –2934 .
-
-
Note that we use here the term ‘double layer’ for electrochemical capacitive processes although the classical Gouy–Chapman–Stern double layer model (see ref. 4) applies for planar electrodes. More complex models may have to be considered for porous electrodes
-
-
- Delahay P., Double Layer and Electrode Kinetics, Wiley, New York, 1955, ch. 7.
-
- Savéant J.-M., Elements of molecular and biomolecular electrochemistry: an electrochemical approach to electron transfer chemistry, John Wiley & Sons, Hoboken, NJ, 2006.
Publication types
LinkOut - more resources
Full Text Sources

