Photoacoustic clinical imaging
- PMID: 31293884
- PMCID: PMC6595011
- DOI: 10.1016/j.pacs.2019.05.001
Photoacoustic clinical imaging
Abstract
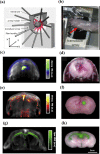
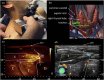
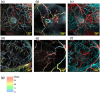
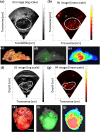
Photoacoustic is an emerging biomedical imaging modality, which allows imaging optical absorbers in the tissue by acoustic detectors (light in - sound out). Such a technique has an immense potential for clinical translation since it allows high resolution, sufficient imaging depth, with diverse endogenous and exogenous contrast, and is free from ionizing radiation. In recent years, tremendous developments in both the instrumentation and imaging agents have been achieved. These opened avenues for clinical imaging of various sites allowed applications such as brain functional imaging, breast cancer screening, diagnosis of psoriasis and skin lesions, biopsy and surgery guidance, the guidance of tumor therapies at the reproductive and urological systems, as well as imaging tumor metastases at the sentinel lymph nodes. Here we survey the various clinical and pre-clinical literature and discuss the potential applications and hurdles that still need to be overcome.
Figures





References
-
- Weissleder R. PMPH-USA; 2010. Molecular Imaging: Principles and Practice.
-
- Park S., Lee C., Kim J., Kim C. Acoustic resolution photoacoustic microscopy. Biomed. Eng. Lett. 2014;4:213–222.
-
- Yoo B., Pagel M.D. An overview of responsive MRI contrast agents for molecular imaging. Front. Biosci. 2008;13:1733–1752. - PubMed
-
- James M.L., Gambhir S.S. A molecular imaging primer: modalities, imaging agents, and applications. Physiol. Rev. 2012;92:897–965. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

