An Auxiliary Approach for the Stereoselective Synthesis of Topologically Chiral Catenanes
- PMID: 31294128
- PMCID: PMC6588264
- DOI: 10.1016/j.chempr.2019.03.008
An Auxiliary Approach for the Stereoselective Synthesis of Topologically Chiral Catenanes
Abstract
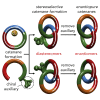
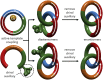
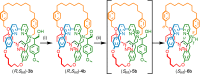
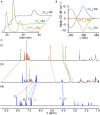
Catenanes, molecules in which two rings are threaded through one another like links in a chain, can form as two structures related like an object and its mirror image but otherwise identical if the individual rings lack bilateral symmetry. These structures are described as "topologically chiral" because, unlike most chiral molecules, it is not possible to convert one mirror-image form to the other under the rules of mathematical topology. Although intriguing and discussed as early as 1961, to date all methods of accessing molecules containing only this topological stereogenic element require the separation of the mirror-image forms via chiral stationary phase high-performance liquid chromatography, which has limited their investigation to date. Here, we present a simple method that uses a readily available source of chiral information to allow the stereoselective synthesis of topologically chiral catenanes.
Keywords: SDG9: Industry, innovation, and infrastructure; catenanes; chirality; mechanical bond; stereoselective; topology.
Figures


References
-
-
1,126 of 11,712 articles published in the Journal of the American Chemical Society, Angewandte Chemie, Chemical Science, Chemical Communications, and Chemistry: A European Journal in 2017 referred to “chiral” or “enantio” in the title, abstract, or keywords (source: Scopus).
-
-
- Pasteur L. Mémoire sur la relation qui peut exister entre la forme cristalline et la composition chimique, et sur la cause de la polarisation rotatoire. C. R. Séances Acad. Sci. 1848;26:535–538.
-
- LeBel J.A. Sur les relations qui existent entre les formules atomiques des corps organiques et le pouvoir rotatoire de leurs dissolutions. Bull. Soc. Chim. Fr. 1874;22:337–347.
-
- van ‘t Hoff J.H. Sur les formules de structure dans l’espace. Arch. Neerl. 1874:1–10.
-
- Mislow K., Siegel J. Stereoisomerism and local chirality. J. Am. Chem. Soc. 1984;106:3319–3328. It should be noted that the presence of a stereogenic unit is a necessary but not sufficient condition because the appearance of chirality as molecular asymmetry is a whole molecule property:
