Effectiveness of three different luteinizing hormone-releasing hormone agonists in the chemical castration of patients with prostate cancer: Goserelin versus triptorelin versus leuprolide
- PMID: 31294133
- PMCID: PMC6607074
- DOI: 10.4111/icu.2019.60.4.244
Effectiveness of three different luteinizing hormone-releasing hormone agonists in the chemical castration of patients with prostate cancer: Goserelin versus triptorelin versus leuprolide
Abstract
Purpose: To investigate the changes in testosterone levels and rates of chemical castration following androgen-deprivation therapy (ADT) with goserelin, triptorelin, and leuprolide.
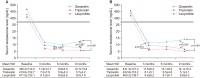
Materials and methods: We retrospectively reviewed the medical records of 125 patients with prostate cancer treated with luteinizing hormone-releasing hormone (LHRH) agonists between January 2009 and December 2015. Changes in testosterone concentration during 9 months of ADT with goserelin 11.34 mg, triptorelin 11.25 mg, and leuprolide 11.25 mg were analyzed using a mixed model. The number of patients with serum testosterone below castration levels defined as various values (<50 ng/dL, <20 ng/dL, or <10 ng/dL) at 3, 6, and 9 months were also evaluated.
Results: Of the 125 patients, 59 received goserelin, 44 received triptorelin, and 22 received leuprolide, respectively. The lowest mean testosterone levels during 9 months of treatment were achieved in patients treated with triptorelin, followed by those treated with leuprolide, and then by those treated with goserelin (p=0.001). Significant differences in chemical castration levels were observed only at <10 ng/dL, with 54.2% of goserelin, 93.2% of triptorelin, and 86.4% of leuprolide treated patients (p<0.001).
Conclusions: Three LHRH agonists showed comparable efficacy for achieving castration when the castration threshold was 50 or 20 ng/dL. However, triptorelin was the most potent LHRH agonist, achieving the lowest mean testosterone levels and the highest rate of chemical castration at <10 ng/dL testosterone.
Keywords: Antineoplastic agents; Prostate-specific antigen; Prostatic neoplasms; Testosterone.
Conflict of interest statement
CONFLICTS OF INTEREST: The authors have nothing to disclose.
Figures
References
-
- Denis L, Murphy GP. Overview of phase III trials on combined androgen treatment in patients with metastatic prostate cancer. Cancer. 1993;72(12 Suppl):3888–3895. - PubMed
-
- Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med. 2000;132:566–577. - PubMed
-
- Dias Silva É, Ferreira U, Matheus W, Faria EF, Silva GD, Saito M, et al. Goserelin versus leuprolide in the chemical castration of patients with prostate cancer. Int Urol Nephrol. 2012;44:1039–1044. - PubMed
-
- Schally AV, Arimura A, Kastin AJ, Matsuo H, Baba Y, Redding TW, et al. Gonadotropin-releasing hormone: one polypeptide regulates secretion of luteinizing and follicle-stimulating hormones. Science. 1971;173:1036–1038. - PubMed
-
- Kiesel L. Molecular mechanisms of gonadotrophin releasing hormone-stimulated gonadotrophin secretion. Hum Reprod. 1993;8(Suppl 2):23–28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical