Phage "delay" towards enhancing bacterial escape from biofilms: a more comprehensive way of viewing resistance to bacteriophages
- PMID: 31294157
- PMCID: PMC6605007
- DOI: 10.3934/microbiol.2017.2.186
Phage "delay" towards enhancing bacterial escape from biofilms: a more comprehensive way of viewing resistance to bacteriophages
Abstract
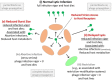
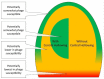
In exploring bacterial resistance to bacteriophages, emphasis typically is placed on those mechanisms which completely prevent phage replication. Such resistance can be detected as extensive reductions in phage ability to form plaques, that is, reduced efficiency of plating. Mechanisms include restriction-modification systems, CRISPR/Cas systems, and abortive infection systems. Alternatively, phages may be reduced in their "vigor" when infecting certain bacterial hosts, that is, with phages displaying smaller burst sizes or extended latent periods rather than being outright inactivated. It is well known, as well, that most phages poorly infect bacteria that are less metabolically active. Extracellular polymers such as biofilm matrix material also may at least slow phage penetration to bacterial surfaces. Here I suggest that such "less-robust" mechanisms of resistance to bacteriophages could serve bacteria by slowing phage propagation within bacterial biofilms, that is, delaying phage impact on multiple bacteria rather than necessarily outright preventing such impact. Related bacteria, ones that are relatively near to infected bacteria, e.g., roughly 10+ µm away, consequently may be able to escape from biofilms with greater likelihood via standard dissemination-initiating mechanisms including erosion from biofilm surfaces or seeding dispersal/central hollowing. That is, given localized areas of phage infection, so long as phage spread can be reduced in rate from initial points of contact with susceptible bacteria, then bacterial survival may be enhanced due to bacteria metaphorically "running away" to more phage-free locations. Delay mechanisms-to the extent that they are less specific in terms of what phages are targeted-collectively could represent broader bacterial strategies of phage resistance versus outright phage killing, the latter especially as require specific, evolved molecular recognition of phage presence. The potential for phage delay should be taken into account when developing protocols of phage-mediated biocontrol of biofilm bacteria, e.g., as during phage therapy of chronic bacterial infections.
Keywords: abortive infection systems; bacteriophage therapy; biofilm; central hollowing; dissemination; extracellular polymeric substances; microcolony; native dispersion; phage resistance; phage therapy; reduced infection vigor; seeding dispersal.
Conflict of interest statement
Conflict of Interest: The author has advised companies with phage therapy interests and maintains the websites phage.org and phage-therapy.org, but received no support in the writing of this manuscript.
Figures




References
LinkOut - more resources
Full Text Sources
