Impact of Adalimumab Patient Support Program's Care Coach Calls on Clinical Outcomes in Patients with Crohn's Disease in Canada: An Observational Retrospective Cohort Study
- PMID: 31294360
- PMCID: PMC6542296
- DOI: 10.1093/jcag/gwy059
Impact of Adalimumab Patient Support Program's Care Coach Calls on Clinical Outcomes in Patients with Crohn's Disease in Canada: An Observational Retrospective Cohort Study
Abstract
Background: Adalimumab is an antitumour necrosis factor (TNFα) biologic therapy indicated for the treatment of Crohn's disease (CD). Patients receiving adalimumab in Canada are eligible to enroll in the AbbVie Care™ patient support program (AC-PSP), which provides personalized services, including care coach calls (CCCs). The objective of this study was to compare the likelihood of achieving clinical remission in a cohort of CD patients treated with adalimumab who did and did not receive CCCs.
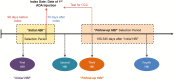
Methods: A longitudinal analysis was performed using de-identified aggregate-level data collected through the AC-PSP. Patients were indexed on the date of their first injection of adalimumab between July 2010 and October 2014. The AC-PSP database included measurements of the Harvey-Bradshaw Index (HBI), a symptom-based measure of disease severity. Eligible patients had an initial HBI measurement performed between 90 days before and up to 30 days after the index date and a follow-up HBI measurement six to 18 months later. Adjusted relative risk (RR) of achieving remission (HBI ≤ 4) at the time of the follow-up was estimated comparing patients who received and did not receive CCCs.
Results: There were 381 CD patients who met eligibility criteria; 224 (59%) received CCCs, and 157 (41%) did not receive CCCs. Multivariate regression analysis demonstrated that CD patients receiving CCCs had a 17% increased likelihood of achieving HBI remission when compared with patients who did not receive CCCs (RR = 1.17; 95% CI, 1.03-1.34; P = 0.0192).
Conclusions: This study provides preliminary evidence that a phone call intervention, aiming to improve the overall patient experience with adalimumab treatment, may increase the likelihood of HBI remission in patients taking adalimumab to manage CD.
Keywords: Adalimumab; Biologics; Crohn’s disease; Inflammatory bowel disease; Patient support programs.
Figures

References
-
- Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet 2012;380(9853):1590–605. - PubMed
-
- Sauter B, Beglinger C, Girardin M, et al. . Monitoring disease activity and progression in Crohn’s disease. A Swiss perspective on the IBD ahead ‘optimised monitoring’ recommendations. Digestion 2014;89(4):299–309. - PubMed
-
- Vermeire S, Schreiber S, Sandborn WJ, et al. . Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol 2010;8(4):357–63. - PubMed
-
- Foti PV, Farina R, Coronella M, et al. . Crohn’s disease of the small bowel: Evaluation of ileal inflammation by diffusion-weighted MR imaging and correlation with the Harvey-Bradshaw index. Radiol Med 2015;120(7):585–94. - PubMed
-
- Walsh AJ, Bryant RV, Travis SP. Current best practice for disease activity assessment in IBD. Nat Rev Gastroenterol Hepatol 2016;13(10):567–79. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
