Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial
- PMID: 31294762
- PMCID: PMC6624820
- DOI: 10.1001/jamaoncol.2019.1449
Pembrolizumab After Completion of Locally Ablative Therapy for Oligometastatic Non-Small Cell Lung Cancer: A Phase 2 Trial
Abstract
Importance: Patients with oligometastatic non-small cell lung cancer (NSCLC) may benefit from locally ablative therapy (LAT) such as surgery or stereotactic radiotherapy. Prior studies were conducted before the advent of immunotherapy, and a strong biological rationale for the use of immunotherapy exists in a minimal residual disease state.
Objective: To evaluate whether the addition of pembrolizumab after LAT improves outcomes for patients with oligometastatic NSCLC.
Design, setting, and participants: This single-arm phase 2 trial of pembrolizumab therapy was performed from February 1, 2015, through September 30, 2017, at an academic referral cancer center. The 51 eligible patients enrolled had oligometastatic NSCLC (≤4 metastatic sites) and had completed LAT to all known sites of disease. Data were analyzed from February 1, 2015, to August 23, 2018.
Interventions: Within 4 to 12 weeks of completing LAT, patients began intravenous pembrolizumab therapy, 200 mg every 21 days, for 8 cycles, with provision to continue to 16 cycles in the absence of progressive disease or untoward toxic effects.
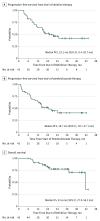
Main outcomes and measures: The 2 primary efficacy end points were progression-free survival (PFS) from the start of LAT (PFS-L), which preceded enrollment in the trial, and PFS from the start of pembrolizumab therapy (PFS-P). The study was powered for comparison with historical data on the first efficacy end point. Secondary outcomes included overall survival, safety, and quality of life as measured by the Functional Assessment of Cancer Therapy-Lung instrument.
Results: Of 51 patients enrolled, 45 (24 men [53%]; median age, 64 years [range, 46-82 years]) received pembrolizumab. At the time of analysis, 24 patients had progressive disease or had died. Median PFS-L was 19.1 months (95% CI, 9.4-28.7 months), significantly greater than the historical median of 6.6 months (P = .005). Median PFS-P was 18.7 months (95% CI, 10.1-27.1 months). Eleven patients died. Overall mean (SE) survival rate at 12 months was 90.9% (4.3%); at 24 months, 77.5% (6.7%). Neither programmed death ligand 1 expression nor CD8 T-cell tumor infiltration was associated with PFS-L. Pembrolizumab after LAT yielded no new safety signals and no reduction in quality of life.
Conclusions and relevance: Pembrolizumab after LAT for oligometastatic NSCLC appears to improve PFS with no reduction in quality of life.
Trial registration: ClinicalTrials.gov identifier: NCT02316002.
Conflict of interest statement
Figures
Comment in
- doi: 10.1001/jamaoncol.2019.1448
References
-
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. . Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17(12):1672-1682. doi:10.1016/S1470-2045(16)30532-0 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials