Efficacy of Phacoemulsification Alone vs Phacoemulsification With Goniosynechialysis in Patients With Primary Angle-Closure Disease: A Randomized Clinical Trial
- PMID: 31294768
- PMCID: PMC6624811
- DOI: 10.1001/jamaophthalmol.2019.2493
Efficacy of Phacoemulsification Alone vs Phacoemulsification With Goniosynechialysis in Patients With Primary Angle-Closure Disease: A Randomized Clinical Trial
Abstract
Importance: The effectiveness of intraocular pressure (IOP) lowering phacoemulsification combined with goniosynechialysis (GSL) compared with phacoemulsification without GSL remains unknown.
Objective: To compare the IOP outcome after 1 year in patients with synechial primary angle-closure disease (PACD) and cataract who underwent phacoemulsification with intraocular lens implantation (PEI) alone compared with PEI with GSL (PEI-GSL).
Design, setting, and participants: A multicenter randomized clinical trial was conducted from September 29, 2011, to March 16, 2015; data analysis was performed from April 1, 2015, to March 4, 2019. Patients with PACD, defined as primary angle closure or primary angle-closure glaucoma, and at least 90° peripheral anterior synechiae (PAS) with cataract were included. Patients were randomized to undergo PEI alone or PEI-GSL. Patients were followed up for 1 year with standardized evaluations. Intention-to-treat analysis was performed.
Interventions: Phacoemulsification with intraocular lens implantation alone or with GSL.
Main outcomes and measures: Successful control of IOP at 12 months, defined as IOP 21 mm Hg or lower without use of topical IOP-lowering medications and a decrease in IOP of 20% or more from baseline IOP.
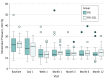
Results: Data from 78 patients (78 eyes) were analyzed. Of these, 37 patients were Chinese (47.4%) and 54 were women (69.2%); mean (SD) age was 67.7 (8.9) years. Mean deviation (SD) at baseline was -13.5 dB (9.4 dB). Forty patients were randomized to the PEI group and 38 to the PEI-GSL group. The mean (SD) IOP at baseline was 22.3 (8.5) mm Hg for the PEI group and 22.9 (5.3) mm Hg for the PEI-GSL group. At 1 year, the mean IOP was 14.3 (5.0) mm Hg for the PEI group and 15.9 (4.5) mm Hg for the PEI-GSL group. Successful control at 1 year occurred in 21 patients (52.5%) in the PEI group and 22 patients (57.9%) in the PEI-GSL group (mean difference, 5.4%; 95% CI, -18.0% to 28.2%; P = .63). In eyes that achieved successful control, mean IOP at 1 year was 12.5 (2.7) mm Hg (range, 7.0-19.0) for the PEI group and 13.6 (2.4) mm Hg (range, 9.0-18.0) for the PEI-GSL group. The number of medications at baseline and 1 year decreased from a mean of 2.2 (0.8) to 0.5 (0.9) in the PEI group and 1.9 (0.9) to 0.6 (1.2) in the PEI-GSL group (P < .001 for each), with a mean change difference of 0.4% (95% CI, -0.02% to 0.9%; P = .06). There were 3 postoperative complications (7.5%) in the PEI group and 3 (7.9%) in the PEI-GSL group. These included IOP spike (IOP≥30 mm Hg) (n = 3), excessive anterior chamber inflammation (n = 1), and posterior capsule opacification (n = 2).
Conclusions and relevance: This randomized clinical trial was unable to show that PEI-GSL added additional IOP lowering compared with PEI alone in patients with PACD.
Trial registration: ClinicalTrials.gov identifier: NCT02376725.
Conflict of interest statement
Figures

Comment in
References
-
- Moghimi S, Lin S. Role of phacoemulsification in angle closure glaucoma. Eye Sci. 2011;26(3):121-131. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

