Mode-of-action analysis of the effects induced by nicotine in the in vitro micronucleus assay
- PMID: 31294873
- PMCID: PMC6900147
- DOI: 10.1002/em.22314
Mode-of-action analysis of the effects induced by nicotine in the in vitro micronucleus assay
Abstract
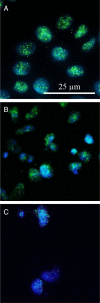
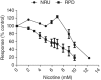
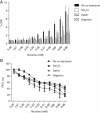
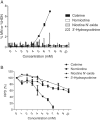
Nicotine's genotoxic potential has been extensively studied in vitro. While the results of mammalian cell-based studies have inferred that it can potentially damage chromosomes, in general and with few exceptions, adverse DNA effects have been observed primarily at supraphysiological concentrations in nonregulatory assays that provide little information on its mode-of-action (MoA). In this study, a modern-day regulatory genotoxicity assessment was conducted using a flow cytometry-based in vitro micronucleus (MN) assay, Good Laboratory Practice study conditions, Chinese hamster ovary cells of known provenance, and acceptance/evaluation criteria from the current OECD Test Guideline 487. Nicotine concentrations up to 3.95 mM had no effect on background levels of DNA damage; however, concentrations above the point-of-departure range of 3.94-4.54 mM induced increases in MN and hypodiploid nuclei, indicating a possible aneugenicity hazard. Follow-up experiments designed to elucidate nicotine's MoA revealed cellular vacuolization, accompanying distortions in microtubules, inhibition of tubulin polymerization, centromere-positive DNA, and multinucleate cells at MN-inducing concentrations. Vacuoles likely originated from acidic cellular compartments (e.g., lysosomes). Remarkably, genotoxicity was suppressed by chemicals that raised the luminal pH of these organelles. Other endpoints (e.g., changes in phosphorylated histones) measured in the study cast doubt on the biological relevance of this apparent genotoxicity. In addition, three major nicotine metabolites, including cotinine, had no MN effects but nornicotine induced a nicotine-like profile. It is possible that nicotine's lysosomotropic properties drive the genotoxicity observed in vitro; however, the potency and mechanistic insights revealed here indicate that it is likely of minimal physiological relevance for nicotine consumers. Environ. Mol. Mutagen. 2019. © 2019 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Keywords: aneugenicity; genotoxic mechanism; lysosomotropism.
© 2019 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals, Inc. on behalf of Environmental Mutagen Society.
Figures

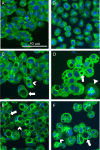
 : vacuole;
: vacuole;  : aberrant metaphase; [
: aberrant metaphase; [ ]: multinuclear cell). (A) Solvent‐treated control. (B) 3.95 mM. (C) 4.93 mM. (D) 5.92 mM. (E) 6.90 mM. (F) 8.88 mM.
]: multinuclear cell). (A) Solvent‐treated control. (B) 3.95 mM. (C) 4.93 mM. (D) 5.92 mM. (E) 6.90 mM. (F) 8.88 mM.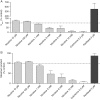

References
-
- Aardema MJ, Albertini S, Arni P, Henderson LM, Kirsch‐Volders M, Mackay JM, Sarrif AM, Stringer DA, Taalman RD. 1998. Aneuploidy: A report of an ECETOC task force. Mutat Res 410:3–79. - PubMed
-
- Adler ID, Attia SM. 2003. Nicotine is not clastogenic at doses of 1 or 2 mg/kg body weight given orally to male mice. Mutat Res 542:139–142. - PubMed
-
- Altmann H, Weniger P, Dolejs I. 1984. Influence of nicotine on DNA metabolism. Klin Wochenschr 62:101–104. - PubMed
-
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, et al. 2010. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–741. - PubMed
-
- Argentin G, Cicchetti R. 2004. Genotoxic and antiapoptotic effect of nicotine on human gingival fibroblasts. Toxicol Sci 79:75–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

