Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: A retrospective study by double immunohistochemistry
- PMID: 31294893
- PMCID: PMC6726681
- DOI: 10.1111/cas.14128
Accurate expression of PD-L1/L2 in lung adenocarcinoma cells: A retrospective study by double immunohistochemistry
Abstract
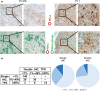
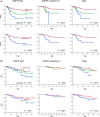
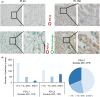
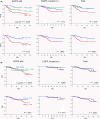
The percentage of programmed death ligand 1 (PD-L1) positivity in cancer cells, named as the tumor proportion score, is considered to be a predictive biomarker for anti-PD-1/PD-L1 therapy in lung cancer. PD-L1 is expressed on not only cancer cells but also on immune cells, including macrophages. Although previous studies related to PD-L1/2 expression in cancer tissues have been generally based on single immunohistochemistry (IHC), in the present study, we attempted to evaluate accurate PD-L1/2 expression in cancer cells in lung adenocarcinoma cells using double IHC to also evaluate macrophages. Of the 231 patients, PD-L1 expression was negative in 169 patients (73.2%), 1%-49% positive in 47 patients (20.3%), and ≥50% positive in 15 patients (6.5%). Interestingly, PD-L1 positivity was decreased when using double IHC compared with the estimation by single IHC. High PD-L1 expression was associated with high-grade cancer cells and in higher stage cancer. PD-L2 was negative in 109 patients (47.2%), 1%-49% positive in 50 patients (21.6%), and ≥50% positive in 72 patients (31.2%). The number of PD-L2-positive patients was increased in cases that had an epidermal growth factor receptor (EGFR) mutation and in lower stage cancer. Thirty-five patients (15.2%) were positive for both PD-L1 and PD-L2, whereas 81 patients (35.1%) were negative for both PD-L1 and PD-L2. Log-rank analysis showed that progression-free survival and overall survival were significantly the longest in the PD-L1-negative and PD-L2-positive groups (P < .0001 and P = .0120). We observed lower PD-L1 or PD-L2 expression in lung adenocarcinoma than previously reported. Double IHC for macrophages may help clinicians to evaluate PD-L1 or PD-L2 expression specifically in cancer cells.
Keywords: PD-L1; PD-L2; lung adenocarcinoma; macrophage; tumor proportion score.
© 2019 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
Similar articles
-
Association between programmed death-ligand 1 expression, immune microenvironments, and clinical outcomes in epidermal growth factor receptor mutant lung adenocarcinoma patients treated with tyrosine kinase inhibitors.Eur J Cancer. 2020 Jan;124:110-122. doi: 10.1016/j.ejca.2019.10.019. Epub 2019 Nov 21. Eur J Cancer. 2020. PMID: 31760310
-
Immune Cell PD-L1 Colocalizes with Macrophages and Is Associated with Outcome in PD-1 Pathway Blockade Therapy.Clin Cancer Res. 2020 Feb 15;26(4):970-977. doi: 10.1158/1078-0432.CCR-19-1040. Epub 2019 Oct 15. Clin Cancer Res. 2020. PMID: 31615933 Free PMC article.
-
PD-L1 expression and T cells infiltration in patients with uncommon EGFR-mutant non-small cell lung cancer and the response to immunotherapy.Lung Cancer. 2020 Apr;142:98-105. doi: 10.1016/j.lungcan.2020.02.010. Epub 2020 Feb 19. Lung Cancer. 2020. PMID: 32120230
-
Prognostic and clinicopathological value of PD-L2 in lung cancer: A meta-analysis.Int Immunopharmacol. 2021 Feb;91:107280. doi: 10.1016/j.intimp.2020.107280. Epub 2020 Dec 25. Int Immunopharmacol. 2021. PMID: 33370681
-
[Efficacy of PD-1/PD-L1 immune checkpoint inhibitors and PD-L1 testing in thoracic cancers].Ann Pathol. 2017 Feb;37(1):61-78. doi: 10.1016/j.annpat.2016.12.009. Epub 2017 Feb 3. Ann Pathol. 2017. PMID: 28162296 Review. French.
Cited by
-
Programmed cell death-ligand 2: A neglected but important target in the immune response to cancer?Transl Oncol. 2020 Oct;13(10):100811. doi: 10.1016/j.tranon.2020.100811. Epub 2020 Jul 1. Transl Oncol. 2020. PMID: 32622310 Free PMC article. Review.
-
IL-32 production from lung adenocarcinoma cells is potentially involved in immunosuppressive microenvironment.Med Mol Morphol. 2024 Jun;57(2):91-100. doi: 10.1007/s00795-023-00378-5. Epub 2024 Feb 6. Med Mol Morphol. 2024. PMID: 38316697
-
SPP1 Derived from Macrophages Is Associated with a Worse Clinical Course and Chemo-Resistance in Lung Adenocarcinoma.Cancers (Basel). 2022 Sep 8;14(18):4374. doi: 10.3390/cancers14184374. Cancers (Basel). 2022. PMID: 36139536 Free PMC article.
-
A Case of Aggressive Lung Squamous Cell Carcinoma With Aberrant Cytoplasmic p53 Aggregation.Cancer Diagn Progn. 2024 Mar 3;4(2):204-208. doi: 10.21873/cdp.10309. eCollection 2024 Mar-Apr. Cancer Diagn Progn. 2024. PMID: 38434916 Free PMC article.
-
Efficacy of Immune Checkpoint Blockade and Biomarkers of Response in Lymphoma: A Narrative Review.Biomedicines. 2023 Jun 15;11(6):1720. doi: 10.3390/biomedicines11061720. Biomedicines. 2023. PMID: 37371815 Free PMC article. Review.
References
-
- Poirier AE, Ruan Y, Walter SD, et al. ComPARe Study Team. The future burden of cancer in Canada: long‐term cancer incidence projections 2013‐2042. Cancer Epidemiol. 2019;59:199‐207. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7‐34. - PubMed
-
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): a multicentre, open‐label, phase 2 randomised controlled trial. Lancet. 2016;387:1837‐1846. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

