Reproduction disrupts stem cell homeostasis in testes of aged male Drosophila via an induced microenvironment
- PMID: 31295251
- PMCID: PMC6622487
- DOI: 10.1371/journal.pgen.1008062
Reproduction disrupts stem cell homeostasis in testes of aged male Drosophila via an induced microenvironment
Abstract
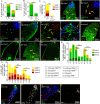
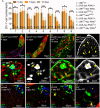
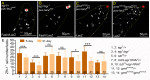
Stem cells rely on instructive cues from their environment. Alterations in microenvironments might contribute to tissue dysfunction and disease pathogenesis. Germline stem cells (GSCs) and cyst stem cells (CySC) in Drosophila testes are normally maintained in the apical area by the testicular hub. In this study, we found that reproduction leads to accumulation of early differentiating daughters of CySCs and GSCs in the testes of aged male flies, due to hyperactivation of Jun-N-terminal kinase (JNK) signaling to maintain self-renewal gene expression in the differentiating cyst cells. JNK activity is normally required to maintain CySCs in the apical niche. A muscle sheath surrounds the Drosophila testis to maintain its long coiled structure. Importantly, reproduction triggers accumulation of the tumor necrosis factor (TNF) Eiger in the testis muscle to activate JNK signaling via the TNF receptor Grindelwald in the cyst cells. Reducing Eiger activity in the testis muscle sheath suppressed reproduction-induced differentiation defects, but had little effect on testis homeostasis of unmated males. Our results reveal that reproduction in males provokes a dramatic shift in the testicular microenvironment, which impairs tissue homeostasis and spermatogenesis in the testes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures









Comment in
-
Irreversible effects of youthful choices in aged adults.PLoS Genet. 2019 Jul 11;15(7):e1008218. doi: 10.1371/journal.pgen.1008218. eCollection 2019 Jul. PLoS Genet. 2019. PMID: 31295252 Free PMC article. No abstract available.
Similar articles
-
Upregulated TNF/Eiger signaling mediates stem cell recovery and tissue homeostasis during nutrient resupply in Drosophila testis.Sci Rep. 2020 Jul 15;10(1):11674. doi: 10.1038/s41598-020-68313-7. Sci Rep. 2020. PMID: 32669615 Free PMC article.
-
Occluding Junctions Maintain Stem Cell Niche Homeostasis in the Fly Testes.Curr Biol. 2016 Sep 26;26(18):2492-2499. doi: 10.1016/j.cub.2016.07.012. Epub 2016 Aug 18. Curr Biol. 2016. PMID: 27546574
-
The Drosophila BCL6 homolog Ken and Barbie promotes somatic stem cell self-renewal in the testis niche.Dev Biol. 2012 Aug 15;368(2):181-92. doi: 10.1016/j.ydbio.2012.04.034. Epub 2012 May 8. Dev Biol. 2012. PMID: 22580161 Free PMC article.
-
Hedgehog in the Drosophila testis niche: what does it do there?Protein Cell. 2013 Sep;4(9):650-5. doi: 10.1007/s13238-013-3040-y. Epub 2013 Jun 26. Protein Cell. 2013. PMID: 23807635 Free PMC article. Review.
-
Signaling Pathways in Drosophila gonadal Stem Cells.Curr Stem Cell Res Ther. 2024;19(2):154-165. doi: 10.2174/1574888X18666230213144531. Curr Stem Cell Res Ther. 2024. PMID: 36788694 Review.
Cited by
-
Signals from the niche promote distinct modes of translation initiation to control stem cell differentiation and renewal in the Drosophila testis.PLoS Biol. 2025 Mar 11;23(3):e3003049. doi: 10.1371/journal.pbio.3003049. eCollection 2025 Mar. PLoS Biol. 2025. PMID: 40067813 Free PMC article.
-
UBR4 deficiency causes male sterility and testis abnormal in Drosophila.Front Endocrinol (Lausanne). 2023 Jul 10;14:1165825. doi: 10.3389/fendo.2023.1165825. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37529615 Free PMC article.
-
Single-cell transcriptome characteristics of testicular terminal epithelium lineages during aging in the Drosophila.Aging Cell. 2024 Mar;23(3):e14057. doi: 10.1111/acel.14057. Epub 2023 Dec 3. Aging Cell. 2024. PMID: 38044573 Free PMC article.
-
Mating-induced ecdysone in the testis disrupts soma-germline contacts and stem cell cytokinesis.bioRxiv [Preprint]. 2023 Nov 10:2023.10.16.562562. doi: 10.1101/2023.10.16.562562. bioRxiv. 2023. Update in: Development. 2024 Jun 1;151(11):dev202542. doi: 10.1242/dev.202542. PMID: 37905121 Free PMC article. Updated. Preprint.
-
Single-cell RNA sequencing reveals cell landscape following antimony exposure during spermatogenesis in Drosophila testes.Cell Death Discov. 2023 Mar 9;9(1):86. doi: 10.1038/s41420-023-01391-4. Cell Death Discov. 2023. PMID: 36894529 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

