Use and validity of child neurodevelopment outcome measures in studies on prenatal exposure to psychotropic and analgesic medications - A systematic review
- PMID: 31295318
- PMCID: PMC6622545
- DOI: 10.1371/journal.pone.0219778
Use and validity of child neurodevelopment outcome measures in studies on prenatal exposure to psychotropic and analgesic medications - A systematic review
Abstract
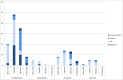
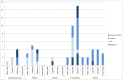
In recent years there has been increased attention to child neurodevelopment in studies on medication safety in pregnancy. Neurodevelopment is a multifactorial outcome that can be assessed by various assessors, using different measures. This has given rise to a debate on the validity of various measures of neurodevelopment. The aim of this review was twofold. Firstly we aimed to give an overview of studies on child neurodevelopment after prenatal exposure to central nervous system acting medications using psychotropics and analgesics as examples, giving special focus on the use and validity of outcome measures. Secondly, we aimed to give guidance on how to conduct and interpret medication safety studies with neurodevelopment outcomes. We conducted a systematic review in the MEDLINE, Embase, PsycINFO, Web of Science, Scopus, and Cochrane databases from inception to April 2019, including controlled studies on prenatal exposure to psychotropics or analgesics and child neurodevelopment, measured with standardised psychometric instruments or by diagnosis of neurodevelopmental disorder. The review management tool Covidence was used for data-extraction. Outcomes were grouped as motor skills, cognition, behaviour, emotionality, or "other". We identified 110 eligible papers (psychotropics, 82 papers, analgesics, 29 papers). A variety of neurodevelopmental outcome measures were used, including 27 different psychometric instruments administered by health care professionals, 15 different instruments completed by parents, and 13 different diagnostic categories. In 23 papers, no comments were made on the validity of the outcome measure. In conclusion, establishing neurodevelopmental safety includes assessing a wide variety of outcomes important for the child's daily functioning including motor skills, cognition, behaviour, and emotionality, with valid and reliable measures from infancy through to adolescence. Consensus is needed in the scientific community on how neurodevelopment should be assessed in medication safety in pregnancy studies. Review registration number: CRD42018086101 in the PROSPERO database.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Broussard CS, Frey MT, Hernandez-Diaz S, Greene MF, Chambers CD, Sahin L, et al. Developing a systematic approach to safer medication use during pregnancy: summary of a Centers for Disease Control and Prevention–convened meeting. Am J Obstet Gynecol. 2014. September;211(3):208–214.e1. 10.1016/j.ajog.2014.05.040 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

