Cost-effectiveness analysis of empagliflozin treatment in people with Type 2 diabetes and established cardiovascular disease in the EMPA-REG OUTCOME trial
- PMID: 31295358
- PMCID: PMC6851686
- DOI: 10.1111/dme.14076
Cost-effectiveness analysis of empagliflozin treatment in people with Type 2 diabetes and established cardiovascular disease in the EMPA-REG OUTCOME trial
Abstract
Aim: In the EMPA-REG OUTCOME trial, empagliflozin therapy reduced cardiovascular death by 38% compared with placebo when added to standard of care. Using the trial results, we created a discrete-event simulation model to assess lifetime health economic outcomes in people with Type 2 diabetes and established cardiovascular disease.
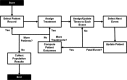
Methods: Time-dependent survival regression analysis was performed on data from EMPA-REG OUTCOME for 10 cardiovascular and renal events (e.g. stroke, heart failure hospitalization, macroalbuminuria, cardiovascular mortality) to capture event rates over time, and interaction between events. Model performance was assessed by comparing predicted and observed outcomes at 3 years. Costs in the United Kingdom (UK) and health utilities were obtained from published literature. Outcomes included cumulative event rates, life-years, costs and quality-adjusted life-years (QALYs).
Results: The model predicted an 18% relative increase (by 2.1 life-years) in survival for empagliflozin (14.0 life-years) vs. standard of care (11.9 life-years), attributable to direct treatment effect on cardiovascular mortality, and to indirect effect via reductions in other events. Participants treated with empagliflozin may experience improved quality of life (1.0 QALY) and higher costs (£3737/participant), yielding an incremental cost-effectiveness ratio (ICER) of £4083/QALY. Sensitivity analyses confirmed the robustness of these results to changes in input parameters.
Conclusions: Based on extrapolation of EMPA-REG OUTCOME trial data using a participant-level simulation model, empagliflozin in addition to standard of care is projected to be highly cost-effective using UK healthcare costs. The impact in other countries will vary due to differences in drug pricing and accrual of other costs. (Clinical Trial Registry No: NCT01131676).
© 2019 The Authors. Diabetic Medicine published by John Wiley & Sons Ltd on behalf of Diabetes UK.
Figures
References
-
- Diabetes mellitus: a major risk factor for cardiovascular disease . A joint editorial statement by the American Diabetes Association; The National Heart, Lung, and Blood Institute; The Juvenile Diabetes Foundation International; The National Institute of Diabetes and Digestive and Kidney Diseases; and The American Heart Association. Circulation 1999; 100: 1132–1133. - PubMed
-
- American Heart Association . Cardiovascular Disease and Diabetes, 2015. Available at http://www.heart.org/HEARTORG/Conditions/Diabetes/WhyDiabetesMatters/Car... Last accessed 12 August 2016.
-
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV et al Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation 1999; 100: 1134–1146. - PubMed
-
- American Diabetes Association . Standards of medical care in diabetes – 2016. Diabetes Care 2016; 39: S1–S112. - PubMed
-
- Tandon N, Ali MK, Narayan KM. Pharmacologic prevention of microvascular and macrovascular complications in diabetes mellitus: implications of the results of recent clinical trials in type 2 diabetes. Am J Cardiovasc Drugs 2012; 12: 7–22. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical