Absence of Dipeptidyl Peptidase 3 Increases Oxidative Stress and Causes Bone Loss
- PMID: 31295380
- PMCID: PMC7203631
- DOI: 10.1002/jbmr.3829
Absence of Dipeptidyl Peptidase 3 Increases Oxidative Stress and Causes Bone Loss
Abstract
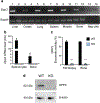
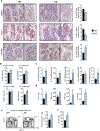
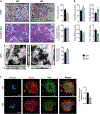
Controlling oxidative stress through the activation of antioxidant pathways is crucial in bone homeostasis, and impairments of the cellular defense systems involved contribute to the pathogenesis of common skeletal diseases. In this work we focused on the dipeptidyl peptidase 3 (DPP3), a poorly investigated ubiquitous zinc-dependent exopeptidase activating the Keap1-Nrf2 antioxidant pathway. We showed Dpp3 expression in bone and, to understand its role in this compartment, we generated a Dpp3 knockout (KO) mouse model and specifically investigated the skeletal phenotype. Adult Dpp3 KO mice showed a mild growth defect, a significant increase in bone marrow cellularity, and bone loss mainly caused by increased osteoclast activity. Overall, in the mouse model, lack of DPP3 resulted in sustained oxidative stress and in alterations of bone microenvironment favoring the osteoclast compared to the osteoblast lineage. Accordingly, in vitro studies revealed that Dpp3 KO osteoclasts had an inherent increased resorptive activity and ROS production, which on the other hand made them prone to apoptosis. Moreover, absence of DPP3 augmented bone loss after estrogen withdrawal in female mice, further supporting its relevance in the framework of bone pathophysiology. Overall, we show a nonredundant role for DPP3 in the maintenance of bone homeostasis and propose that DPP3 might represent a possible new osteoimmunological player and a marker of human bone loss pathology. © 2019 American Society for Bone and Mineral Research.
Keywords: BONE LOSS; DPP3; OSTEOPOROSIS; OXIDATIVE STRESS.
© 2019 American Society for Bone and Mineral Research.
Figures


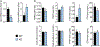


References
-
- Kenkre JS, Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55(3):308–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

