Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer's disease-like pathology
- PMID: 31295467
- PMCID: PMC7014559
- DOI: 10.1016/j.brainres.2019.146327
Over-expression of miR-34a induces rapid cognitive impairment and Alzheimer's disease-like pathology
Abstract
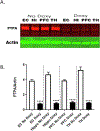
Autosomal dominant Alzheimer disease (AD) is caused by rare mutations in one of three specific genes. This is in contrast to idiopathic, late-onset AD (LOAD), which has a more polygenetic risk profile and represents more than 95% of cases. Previously, we have demonstrated that increased expression of microRNA (miRNA)-34a (miR-34a) in AD brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity. Here we report the generation of a heterozygous, conditional miR-34a overexpression mouse (miR-34a+/-(TetR-TetO-miR-34a) Transgenic Mice). Doxycycline-treated mice of either sex exhibited profound behavioral impairment compared to untreated groups with only 1-2 months of over-expression of miR-34a. Cognitive impairment of individual mice in T- and Y-maze tasks correlated with elevated miR-34a expression in many parts of the brain including the hippocampus and prefrontal cortex, regions which are known to be involved in this task and implicated in LOAD dysfunction. Immunocytochemistry of brain sections from mice show high amyloid β and phosphorylated tau-specific staining in the hippocampus and cortex. Analysis of protein samples from these mice revealed that miR-34a targets specific genes involved in memory formation, amyloid precursor protein (APP) metabolism and phosphorylation-dephosphorylation of tau. Thus, our results suggest that the polygenetic dysfunction caused by miR-34a may occur in LOAD and disclose miR-34a as a potential therapeutic target. SIGNIFICANCE STATEMENT: Late-onset Alzheimer disease (LOAD) is associated with multiple gene alleles, a polygenetic profile of risk factors that is difficult to model in animals. Our approach to modeling LOAD was to produce a conditional over-expressing, miR-34a mouse using doxycycline-induction to activate expression. We observed that miR-34a over-expression results in a rapid cognitive impairment, associated with accumulation of intracellular Aβ and tau hyperphosphorylation in multiple brain regions. Targets for miR-34a, including ADAM10, NMDAR 2B, and SIRT1 RNAs, were profoundly reduced by miR-34a over-expression. Collectively, these results indicate that a rapid, profound cognitive decline and Alzheimer's disease neuropathology can be induced with miR-34a over-expression, suggesting that this animal model may represent a polygenetic risk factor model for LOAD.
Keywords: ADAM10; Late-onset Alzheimer’s disease; Memory-related behaviors; NMDAR 2B; SIRT1; Tau phosphorylation; miR-34a; microRNA; β amyloid.
Copyright © 2019. Published by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest
The authors declare no conflicts of interest related to this research.
Figures

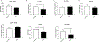
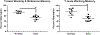



Similar articles
-
Expression of microRNA-34a in Alzheimer's disease brain targets genes linked to synaptic plasticity, energy metabolism, and resting state network activity.Brain Res. 2016 Sep 1;1646:139-151. doi: 10.1016/j.brainres.2016.05.026. Epub 2016 May 25. Brain Res. 2016. PMID: 27235866 Free PMC article.
-
miR-34a deficiency in APP/PS1 mice promotes cognitive function by increasing synaptic plasticity via AMPA and NMDA receptors.Neurosci Lett. 2018 Mar 23;670:94-104. doi: 10.1016/j.neulet.2018.01.045. Epub 2018 Feb 6. Neurosci Lett. 2018. PMID: 29378298
-
Cognitive Decline and Modulation of Alzheimer's Disease-Related Genes After Inhibition of MicroRNA-101 in Mouse Hippocampal Neurons.Mol Neurobiol. 2020 Jul;57(7):3183-3194. doi: 10.1007/s12035-020-01957-8. Epub 2020 Jun 5. Mol Neurobiol. 2020. PMID: 32504417
-
Effects of CX3CR1 and Fractalkine Chemokines in Amyloid Beta Clearance and p-Tau Accumulation in Alzheimer's Disease (AD) Rodent Models: Is Fractalkine a Systemic Biomarker for AD?Curr Alzheimer Res. 2016;13(4):403-12. doi: 10.2174/1567205013666151116125714. Curr Alzheimer Res. 2016. PMID: 26567742 Review.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
Cited by
-
Berberine exerts antidepressant-like effects via regulating miR-34a-synaptotagmin1/Bcl-2 axis.Chin Herb Med. 2020 Dec 1;13(1):116-123. doi: 10.1016/j.chmed.2020.11.001. eCollection 2021 Jan. Chin Herb Med. 2020. PMID: 36117760 Free PMC article.
-
miR-3940-5p reduces amyloid β production via selectively targeting PSEN1.Front Aging Neurosci. 2024 Mar 4;16:1346978. doi: 10.3389/fnagi.2024.1346978. eCollection 2024. Front Aging Neurosci. 2024. PMID: 38501059 Free PMC article.
-
Mitochondrial Dysfunction and Mitophagy Closely Cooperate in Neurological Deficits Associated with Alzheimer's Disease and Type 2 Diabetes.Mol Neurobiol. 2021 Aug;58(8):3677-3691. doi: 10.1007/s12035-021-02365-2. Epub 2021 Apr 1. Mol Neurobiol. 2021. PMID: 33797062 Review.
-
MicroRNAs in Learning and Memory and Their Impact on Alzheimer's Disease.Biomedicines. 2022 Aug 1;10(8):1856. doi: 10.3390/biomedicines10081856. Biomedicines. 2022. PMID: 36009403 Free PMC article. Review.
-
MicroRNAs modulation by curcumin, catalpol, and other natural products in Alzheimer's disease: a review.Mol Biol Rep. 2025 May 6;52(1):445. doi: 10.1007/s11033-025-10543-x. Mol Biol Rep. 2025. PMID: 40327129 Review.
References
-
- Bannon AW, Malmberg AB, 2007. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents. Curr. Protoc. Neurosci 41, 8.9.1–8.9.16. - PubMed
-
- Baron SP, Wenger GR, 2001. Effects of drugs of abuse on response accuracy and bias under a delayed matching-to-sample procedure in squirrel monkeys. Behav. Pharmacol 12, 247–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

