New insights into regulation and function of planar polarity in the inner ear
- PMID: 31295539
- PMCID: PMC6732021
- DOI: 10.1016/j.neulet.2019.134373
New insights into regulation and function of planar polarity in the inner ear
Abstract
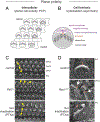
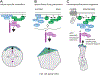
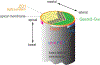
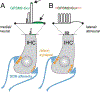
Acquisition of cell polarity generates signaling and cytoskeletal asymmetry and thus underpins polarized cell behaviors during tissue morphogenesis. In epithelial tissues, both apical-basal polarity and planar polarity, which refers to cell polarization along an axis orthogonal to the apical-basal axis, are essential for epithelial morphogenesis and function. A prime example of epithelial planar polarity can be found in the auditory sensory epithelium (or organ of Corti, OC). Sensory hair cells, the sound receptors, acquire a planar polarized apical cytoskeleton which is uniformely oriented along an axis orthogonal to the longitudinal axis of the cochlear duct. Both cell-intrinsic and tissue-level planar polarity are necessary for proper perception of sound. Here we review recent insights into the novel roles and mechanisms of planar polarity signaling gained from genetic analysis in mice, focusing mainly on the OC but also with some discussions on the vestibular sensory epithelia.
Keywords: Cochlea; Deafness; Hair bundle; Hair cell; Kinocilium; Planar cell polarity; Stereocilia.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures


References
-
- Beer-Hammer S, Lee SC, Mauriac SA, Leiss V, Groh IAM, Novakovic A, Piekorz RP, Bucher K, Chen C, Ni K, Singer W, Harasztosi C, Schimmang T, Zimmermann U, Pfeffer K, Birnbaumer L, Forge A, Montcouquiol M, Knipper M, Nurnberg B, Ruttiger L, Galphai Proteins are Indispensable for Hearing, Cell Physiol Biochem 47 (2018) 1509–1532. - PMC - PubMed
-
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB, Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia, Nat Cell Biol 7 (2005) 148–156. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

