Immune Responses in the Eye-Associated Lymphoid Tissues of Chickens after Ocular Inoculation with Vaccine and Virulent Strains of the Respiratory Infectious Laryngotracheitis Virus (ILTV)
- PMID: 31295877
- PMCID: PMC6669519
- DOI: 10.3390/v11070635
Immune Responses in the Eye-Associated Lymphoid Tissues of Chickens after Ocular Inoculation with Vaccine and Virulent Strains of the Respiratory Infectious Laryngotracheitis Virus (ILTV)
Abstract
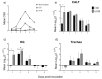
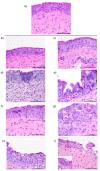
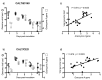
Infectious laryngotracheitis (ILT) is an acute respiratory disease of poultry caused by infectious laryngotracheitis virus (ILTV). Control of the disease with live attenuated vaccines administered via eye drop build upon immune responses generated by the eye-associated lymphoid tissues. The aim of this study was to assess cytokine and lymphocyte changes in the conjunctiva-associated lymphoid tissues (CALT) and Harderian gland (HG) stimulated by the ocular inoculation of the ILTV chicken embryo origin (CEO) vaccine strain and virulent strain 63140. This study offers strong evidence to support the roles that the CALT and HG play in the development of protective ILTV immune responses. It supports the premise that ILTV-mediated immunomodulation favors the B cell response over those of T cells. Further, it provides evidence that expansions of CD8α+ cells, with the concomitant expression of the Granzyme A gene, are key to reducing viral genomes in the CALT and halting ILTV cytolytic replication in the conjunctiva. Ultimately, this study revealed that the early upregulation of interleukin (IL)-12p40 and Interferon (IFN)-γ cytokine genes, which shape the antigen-specific cell-mediated immune responses, retarded the decline of virus replication, and enhanced the development of lesions in the conjunctiva epithelium.
Keywords: Granzyme A; Harderian gland (HG); Interferon-γ gene; conjunctiva-associated lymphoid tissue (CALT); infectious laryngotracheitis virus (ILTV); interferon gamma; interleukin-12p40 gene; viral genome load.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



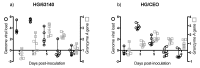

References
-
- García M., Spatz S., Guy J. Infectious laryngotracheitis. In: Swayne D.E., Glisson J.R., McDougland L.R., Nolan L.K., Suarez D.L., Nair V.L., editors. Diseases of Poultry. 13th ed. Wiley-Blackwell; Ames, IA, USA: 2013. pp. 161–179.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

