The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle
- PMID: 31295970
- PMCID: PMC6678760
- DOI: 10.3390/cells8070701
The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle
Abstract
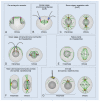
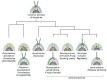
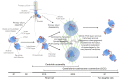
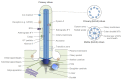
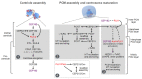
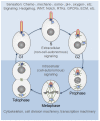
Centrosomes and primary cilia are usually considered as distinct organelles, although both are assembled with the same evolutionary conserved, microtubule-based templates, the centrioles. Centrosomes serve as major microtubule- and actin cytoskeleton-organizing centers and are involved in a variety of intracellular processes, whereas primary cilia receive and transduce environmental signals to elicit cellular and organismal responses. Understanding the functional relationship between centrosomes and primary cilia is important because defects in both structures have been implicated in various diseases, including cancer. Here, we discuss evidence that the animal centrosome evolved, with the transition to complex multicellularity, as a hybrid organelle comprised of the two distinct, but intertwined, structural-functional modules: the centriole/primary cilium module and the pericentriolar material/centrosome module. The evolution of the former module may have been caused by the expanding cellular diversification and intercommunication, whereas that of the latter module may have been driven by the increasing complexity of mitosis and the requirement for maintaining cell polarity, individuation, and adhesion. Through its unique ability to serve both as a plasma membrane-associated primary cilium organizer and a juxtanuclear microtubule-organizing center, the animal centrosome has become an ideal integrator of extracellular and intracellular signals with the cytoskeleton and a switch between the non-cell autonomous and the cell-autonomous signaling modes. In light of this hypothesis, we discuss centrosome dynamics during cell proliferation, migration, and differentiation and propose a model of centrosome-driven microtubule assembly in mitotic and interphase cells. In addition, we outline the evolutionary benefits of the animal centrosome and highlight the hierarchy and modularity of the centrosome biogenesis networks.
Keywords: cell cycle; cell differentiation; cell signaling; centriole; centrosome; microtubule cytoskeleton; microtubule nucleation; mitosis; organelle biogenesis; primary cilia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

