High therapeutic efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Somalia
- PMID: 31296223
- PMCID: PMC6624891
- DOI: 10.1186/s12936-019-2864-1
High therapeutic efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated falciparum malaria in Somalia
Abstract
Background: Artemether-lumefantrine (AL) and dihydroartemisinin-piperaquine (DHA/PPQ) are the recommended first- and second-line treatments, respectively, for uncomplicated falciparum malaria in Somalia. The studies reported here were conducted to assess the efficacy of these artemisinin-based combinations and the mutations in Plasmodium falciparum K13-propeller (Pfk13) domain and amplification in Pfplasmepsin 2 (Pfpm2) gene in Somalia.
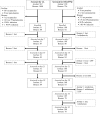
Methods: One-arm prospective studies were conducted to assess the clinical and parasitological responses to DHA/PPQ and AL at two sites in 2016 and 2017, respectively, using the standard WHO protocol. The patterns of molecular markers associated with artemisinin and PPQ resistance were investigated for the first time in Somalia.
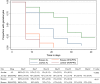
Results: A total of 339 patients were enrolled with 139 for AL and 200 for DHA/PPQ. With AL, no parasite recurrence was observed among patients treated at either site, corresponding to 100% clinical and parasitological responses. For DHA-PPQ, an adequate clinical and parasitological response rate > 97% was observed. All study patients on both treatments at both sites were parasite-free on day 3. Of the 138 samples with interpretable results for the polymorphism in Pfk13, only one (0.7%), from Bosaso, contained a non-synonymous mutation (R622I), which is not one of the known markers of artemisinin resistance. No Pfpm2 amplification was observed among the 135 samples with interpretable results.
Conclusions: AL and DHA/PPQ were highly effective in the treatment of uncomplicated falciparum malaria, and there was no evidence of resistance to artemisinin or PPQ. These two combinations are thus relevant in the chemotherapeutic strategy for malaria control in Somalia. Trial registration ACTRN12616001005448 (Jowhar DP study), ACTRN12616000553471 (Bosaso DP study), ACTRN12617001055392 (AL study in Bosaso and Jowhar).
Keywords: Artemether–lumefantrine; Artemisinin resistance; Dihydroartemisinin–piperaquine; Piperaquine resistance; Plasmodium falciparum; Somalia.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Therapeutic efficacies of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and chloroquine and dihydroartemisinin-piperaquine for uncomplicated Plasmodium vivax infection in Ethiopia.Malar J. 2022 Dec 1;21(1):359. doi: 10.1186/s12936-022-04350-z. Malar J. 2022. PMID: 36451216 Free PMC article. Clinical Trial.
-
Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated malaria in Papua New Guinea.Malar J. 2018 Oct 5;17(1):350. doi: 10.1186/s12936-018-2494-z. Malar J. 2018. PMID: 30290825 Free PMC article. Clinical Trial.
-
Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: a multicentre, open-label, randomised clinical trial.Lancet. 2020 Apr 25;395(10233):1345-1360. doi: 10.1016/S0140-6736(20)30552-3. Epub 2020 Mar 11. Lancet. 2020. PMID: 32171078 Free PMC article. Clinical Trial.
-
Efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria among children in Africa: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Aug 12;20(1):340. doi: 10.1186/s12936-021-03873-1. Malar J. 2021. PMID: 34384431 Free PMC article.
-
Efficacy and safety of dihydroartemisinin-piperaquine versus artemether-lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Ugandan children: a systematic review and meta-analysis of randomized control trials.Malar J. 2021 Apr 1;20(1):174. doi: 10.1186/s12936-021-03711-4. Malar J. 2021. PMID: 33794897 Free PMC article.
Cited by
-
Prevalence of mutations in the cysteine desulfurase IscS (Pfnfs1) gene in recurrent Plasmodium falciparum infections following artemether-lumefantrine (AL) and dihydroartemisinin-piperaquine (DP) treatment in Matayos, Western Kenya.Malar J. 2023 May 19;22(1):158. doi: 10.1186/s12936-023-04587-2. Malar J. 2023. PMID: 37202779 Free PMC article.
-
Therapeutic efficacies of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum and chloroquine and dihydroartemisinin-piperaquine for uncomplicated Plasmodium vivax infection in Ethiopia.Malar J. 2022 Dec 1;21(1):359. doi: 10.1186/s12936-022-04350-z. Malar J. 2022. PMID: 36451216 Free PMC article. Clinical Trial.
-
Therapeutic efficacy of artesunate-amodiaquine and artemether-lumefantrine and polymorphism in Plasmodium falciparum kelch13-propeller gene in Equatorial Guinea.Malar J. 2021 Jun 22;20(1):275. doi: 10.1186/s12936-021-03807-x. Malar J. 2021. PMID: 34158055 Free PMC article.
-
Increasing Prevalence of Artemisinin-Resistant HRP2-Negative Malaria in Eritrea.N Engl J Med. 2023 Sep 28;389(13):1191-1202. doi: 10.1056/NEJMoa2210956. N Engl J Med. 2023. PMID: 37754284 Free PMC article.
-
Progressive heterogeneity of enlarged and irregularly shaped apicoplasts in P. falciparum persister blood stages after drug treatment.bioRxiv [Preprint]. 2024 Aug 29:2024.01.03.574077. doi: 10.1101/2024.01.03.574077. bioRxiv. 2024. Update in: PNAS Nexus. 2024 Sep 24;3(10):pgae424. doi: 10.1093/pnasnexus/pgae424. PMID: 38410435 Free PMC article. Updated. Preprint.
References
-
- WHO. World Malaria Report 2017. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
-
- WHO. Guidelines for the treatment of malaria. 3rd edn. Geneva: World Health Organization; 2015. https://www.who.int/malaria/publications/atoz/9789241500470/en/. - PubMed
-
- WHO. Status report on artemisinin resistance and artemisinin-based combination therapy efficacy (August 2018). Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/atoz/artemisinin-resistance-aug....
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

