K+-Cl- cotransporter 1 (KCC1): a housekeeping membrane protein that plays key supplemental roles in hematopoietic and cancer cells
- PMID: 31296230
- PMCID: PMC6624878
- DOI: 10.1186/s13045-019-0766-x
K+-Cl- cotransporter 1 (KCC1): a housekeeping membrane protein that plays key supplemental roles in hematopoietic and cancer cells
Abstract
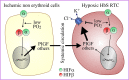
During the 1970s, a Na+-independent, ouabain-insensitive, N-ethylmaleimide-stimulated K+-Cl- cotransport mechanism was identified in red blood cells for the first time and in a variety of cell types afterward. During and just after the mid-1990s, three closely related isoforms were shown to account for this mechanism. They were termed K+-Cl- cotransporter 1 (KCC1), KCC3, and KCC4 according to the nomenclature of Gillen et al. (1996) who had been the first research group to uncover the molecular identity of a KCC, that is, of KCC1 in rabbit kidney. Since then, KCC1 has been found to be the most widely distributed KCC isoform and considered to act as a housekeeping membrane protein. It has perhaps received less attention than the other isoforms for this reason, but as will be discussed in the following review, there is probably more to KCC1 than meets the eye. In particular, the so-called housekeeping gene also appears to play crucial and specific roles in normal as well as pathological hematopoietic and in cancer cells.
Keywords: Abnormal cell growth; Animal models; Cation-Cl− cotransporter; K+-Cl− cotransporter; Red blood cells; Sickle cell anemia; Submitted to Journal of Hematology and Oncology.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Molecular insights into the normal operation, regulation, and multisystemic roles of K+-Cl- cotransporter 3 (KCC3).Am J Physiol Cell Physiol. 2017 Nov 1;313(5):C516-C532. doi: 10.1152/ajpcell.00106.2017. Epub 2017 Aug 16. Am J Physiol Cell Physiol. 2017. PMID: 28814402 Review.
-
Characterization of glial cell K-Cl cotransport.Cell Physiol Biochem. 2007;20(1-4):121-30. doi: 10.1159/000104160. Cell Physiol Biochem. 2007. PMID: 17595522
-
Multiple isoforms of the KC1 cotransporter are expressed in sickle and normal erythroid cells.Exp Hematol. 2005 Jun;33(6):624-31. doi: 10.1016/j.exphem.2005.02.006. Exp Hematol. 2005. PMID: 15911086
-
Functional comparison of the K+-Cl- cotransporters KCC1 and KCC4.J Biol Chem. 2000 Sep 29;275(39):30326-34. doi: 10.1074/jbc.M003112200. J Biol Chem. 2000. PMID: 10913127
-
Regulation of K-Cl cotransport: from function to genes.J Membr Biol. 2004 Oct 1;201(3):109-37. doi: 10.1007/s00232-004-0695-6. J Membr Biol. 2004. PMID: 15711773 Review.
Cited by
-
Gut commensal metabolite rhamnose promotes macrophages phagocytosis by activating SLC12A4 and protects against sepsis in mice.Acta Pharm Sin B. 2024 Jul;14(7):3068-3085. doi: 10.1016/j.apsb.2024.03.025. Epub 2024 Mar 22. Acta Pharm Sin B. 2024. PMID: 39027244 Free PMC article.
-
Erythroid-specific inactivation of Slc12a6/Kcc3 by EpoR promoter-driven Cre expression reduces K-Cl cotransport activity in mouse erythrocytes.Physiol Rep. 2022 Mar;10(5):e15186. doi: 10.14814/phy2.15186. Physiol Rep. 2022. PMID: 35274823 Free PMC article.
-
The biogenesis of potassium transporters: implications of disease-associated mutations.Crit Rev Biochem Mol Biol. 2024 Jun-Aug;59(3-4):154-198. doi: 10.1080/10409238.2024.2369986. Epub 2024 Jul 1. Crit Rev Biochem Mol Biol. 2024. PMID: 38946646 Free PMC article. Review.
-
Signaling pathway mechanisms of neurological diseases induced by G protein-coupled receptor 39.CNS Neurosci Ther. 2023 Jun;29(6):1470-1483. doi: 10.1111/cns.14174. Epub 2023 Mar 21. CNS Neurosci Ther. 2023. PMID: 36942516 Free PMC article. Review.
-
Molecular mechanisms, physiological roles, and therapeutic implications of ion fluxes in bone cells: Emphasis on the cation-Cl- cotransporters.J Cell Physiol. 2022 Dec;237(12):4356-4368. doi: 10.1002/jcp.30879. Epub 2022 Sep 20. J Cell Physiol. 2022. PMID: 36125923 Free PMC article. Review.
References
-
- Garneau A. P., Marcoux A. A., Slimani S., Tremblay L. E., Frenette‐Cotton R., Mac‐Way F., Isenring P. Physiological roles and molecular mechanisms of K + ‐Cl − cotransport in the mammalian kidney and cardiovascular system: where are we? The Journal of Physiology. 2019;597(6):1451–1465. doi: 10.1113/JP276807. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

