Long-term voluntary wheel running does not alter vascular amyloid burden but reduces neuroinflammation in the Tg-SwDI mouse model of cerebral amyloid angiopathy
- PMID: 31296239
- PMCID: PMC6621983
- DOI: 10.1186/s12974-019-1534-0
Long-term voluntary wheel running does not alter vascular amyloid burden but reduces neuroinflammation in the Tg-SwDI mouse model of cerebral amyloid angiopathy
Abstract
Background: Cardiovascular exercise (CVE) has been shown to be protective against cognitive decline in aging and the risk for dementias, including Alzheimer's Disease (AD). CVE has also been shown to have several beneficial effects on brain pathology and behavioral impairments in mouse models of AD; however, no studies have specifically examined the effects of CVE on cerebral amyloid angiopathy (CAA), which is the accumulation of amyloid-beta (Aβ) in the cerebral vasculature. CAA may be uniquely susceptible to beneficial effects of CVE interventions due to the location and nature of the pathology. Alternatively, CVE may exacerbate CAA pathology, due to added stress on already compromised cerebral vasculature.
Methods: In the current study, we examined the effects of CVE over many months in mice, thereby modeling a lifelong commitment to CVE in humans. We assessed this voluntary CVE in Tg-SwDI mice, a transgenic mouse model of CAA that exhibits behavioral deficits, fibrillar vascular Aβ pathology, and significant perivascular neuroinflammation. Various "doses" of exercise intervention (0 h ("Sedentary"), 1 h, 3 h, 12 h access to running wheel) were assessed from ~ 4 to 12 months of age for effects on physiology, behavior/cognitive performance, and pathology.
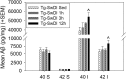
Results: The 12 h group performed the greatest volume of exercise, whereas the 1 h and 3 h groups showed high levels of exercise intensity, as defined by more frequent and longer duration running bouts. Tg-SwDI mice exhibited significant cerebral vascular Aβ pathology and increased expression of pro-inflammatory cytokines as compared to WT controls. Tg-SwDI mice did not show motor dysfunction or altered levels of anxiety or sociability compared to WT controls, though Tg-SwDI animals did appear to exhibit a reduced tendency to explore novel environments. At all running levels, CAA pathology in Tg-SwDI mice was not significantly altered, but 12-h high-volume exercise showed increased insoluble Aβ burden. However, CVE attenuated the expression of pro-inflammatory cytokines TNF-α and IL-6 and was generally effective at enhancing motor function and reducing anxiety-like behavior in Tg-SwDI mice, though alterations in learning and memory tasks were varied.
Conclusions: Taken together, these results suggest that CAA can still develop regardless of a lifespan of substantial CVE, although downstream effects on neuroinflammation may be reduced and functional outcomes improved.
Keywords: Aerobic; Alzheimer’s disease; Anti-inflammatory; Beta amyloid; Cardiovascular; Cerebral amyloid angiopathy; Exercise; Fitness; Inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

