Antibiotic Review Kit for Hospitals (ARK-Hospital): study protocol for a stepped-wedge cluster-randomised controlled trial
- PMID: 31296255
- PMCID: PMC6625068
- DOI: 10.1186/s13063-019-3497-y
Antibiotic Review Kit for Hospitals (ARK-Hospital): study protocol for a stepped-wedge cluster-randomised controlled trial
Abstract
Background: To ensure patients continue to get early access to antibiotics at admission, while also safely reducing antibiotic use in hospitals, one needs to target the continued need for antibiotics as more diagnostic information becomes available. UK Department of Health guidance promotes an initiative called 'Start Smart then Focus': early effective antibiotics followed by active 'review and revision' 24-72 h later. However in 2017, < 10% of antibiotic prescriptions were discontinued at review, despite studies suggesting that 20-30% of prescriptions could be stopped safely.
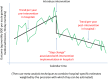
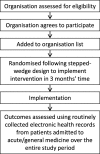
Methods/design: Antibiotic Review Kit for Hospitals (ARK-Hospital) is a complex 'review and revise' behavioural intervention targeting healthcare professionals involved in antibiotic prescribing or administration in inpatients admitted to acute/general medicine (the largest consumers of non-prophylactic antibiotics in hospitals). The primary study objective is to evaluate whether ARK-Hospital can safely reduce the total antibiotic burden in acute/general medical inpatients by at least 15%. The primary hypotheses are therefore that the introduction of the behavioural intervention will be non-inferior in terms of 30-day mortality post-admission (relative margin 5%) for an acute/general medical inpatient, and superior in terms of defined daily doses of antibiotics per acute/general medical admission (co-primary outcomes). The unit of observation is a hospital organisation, a single hospital or group of hospitals organised with one executive board and governance framework (National Health Service trusts in England; health boards in Northern Ireland, Wales and Scotland). The study comprises a feasibility study in one organisation (phase I), an internal pilot trial in three organisations (phase II) and a cluster (organisation)-randomised stepped-wedge trial (phase III) targeting a minimum of 36 organisations in total. Randomisation will occur over 18 months from November 2017 with a further 12 months follow-up to assess sustainability. The behavioural intervention will be delivered to healthcare professionals involved in antibiotic prescribing or administration in adult inpatients admitted to acute/general medicine. Outcomes will be assessed in adult inpatients admitted to acute/general medicine, collected through routine electronic health records in all patients.
Discussion: ARK-Hospital aims to provide a feasible, sustainable and generalisable mechanism for increasing antibiotic stopping in patients who no longer need to receive them at 'review and revise'.
Trial registration: ISRCTN Current Controlled Trials, ISRCTN12674243 . Registered on 10 April 2017.
Keywords: Antibiotic prescribing; Antimicrobial stewardship; Hospitals.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- HM Government: Tackling antimicrobial resistance 2019–2024: The UK's five-year national action plan. 2019. https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... [Accessed 21 June 2019]. - PubMed
-
- World Health Organization (WHO). The evolving threat of antimicrobial resistance: options for action. Geneva: WHO; 2012. https://apps.who.int/iris/handle/10665/44812. [Accessed 21 June 2019].
-
- Cassini A, Hogberg LD, Plachouras D, Quattrocchi A, Hoxha A, Simonsen GS, Colomb-Cotinat M, Kretzschmar ME, Devleesschauwer B, Cecchini M, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. doi: 10.1016/S1473-3099(18)30605-4. - DOI - PMC - PubMed
-
- European Center for Disease Prevention and Control (ECDC). Surveillance of antimicrobial resistance in Europe – Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). 2017. Stockholm: ECDC; 2018. https://www.ecdc.europa.eu/sites/portal/files/documents/EARS-Net-report-.... [Accessed 21 June 2019].
-
- Public Health England (PHE): English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2018. London: PHE; 2018. https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... [Accessed 21 June 2019].
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

