Complement-independent bystander injury in AQP4-IgG seropositive neuromyelitis optica produced by antibody-dependent cellular cytotoxicity
- PMID: 31296268
- PMCID: PMC6621951
- DOI: 10.1186/s40478-019-0766-7
Complement-independent bystander injury in AQP4-IgG seropositive neuromyelitis optica produced by antibody-dependent cellular cytotoxicity
Abstract
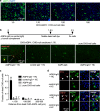
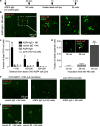
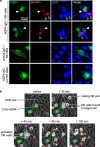
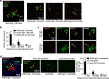
Cellular injury in AQP4-IgG seropositive neuromyelitis spectrum disorder (herein called NMO) involves AQP4-IgG binding to astrocytes, resulting in astrocyte injury by complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) mechanisms. The rapid disease progression, severe tissue damage, and abundant leukocyte infiltration seen in some NMO patients suggest a more direct mechanism for demyelination and neurologic deficit than secondary injury from astrocyte loss. Here, we report evidence for an 'ADCC bystander mechanism' in NMO involving injury to nearby cells by leukocytes following their activation by AQP4-bound AQP4-IgG on astrocytes. In model cocultures containing AQP4-expressing and null CHO cells, AQP4-IgG and complement killed bystander null cells to ~ 100 μm away from AQP4-expressing cells; AQP4-IgG and NK cells produced bystander killing to ~ 300 μm, with perforin deposition seen on injured null cells. Bystander cytotoxicity was also seen with neutrophil-mediated ADCC and in astrocyte-neuron cocultures. Mechanistic studies, including real-time imaging, suggested that leukocytes activated by an AQP4-dependent ADCC mechanism injure bystander cells by direct targeted exocytosis on neighboring cells and not by diffusion of soluble granule contents. In support of this conclusion, ADCC bystander injury was preferentially reduced by an RGDS peptide that inhibits integrin adhesion. Evidence for ADCC bystander injury to oligodendrocytes and neurons was also found in mice following intracerebral injection of AQP4-IgG and NK cells, which was inhibited by RGDS peptide. These results establish a novel cellular pathogenesis mechanism in AQP4-IgG seropositive NMO and provide evidence that inflammatory mechanisms can cause widespread tissue damage in NMO independently of the secondary effects from astrocyte loss.
Keywords: ADCC; Aquaporin-4; Astrocyte; Leukocyte; NMO.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Complement-dependent bystander injury to neurons in AQP4-IgG seropositive neuromyelitis optica.J Neuroinflammation. 2018 Oct 22;15(1):294. doi: 10.1186/s12974-018-1333-z. J Neuroinflammation. 2018. PMID: 30348195 Free PMC article.
-
Bystander mechanism for complement-initiated early oligodendrocyte injury in neuromyelitis optica.Acta Neuropathol. 2017 Jul;134(1):35-44. doi: 10.1007/s00401-017-1734-6. Epub 2017 May 31. Acta Neuropathol. 2017. PMID: 28567523 Free PMC article.
-
Involvement of antibody-dependent cell-mediated cytotoxicity in inflammatory demyelination in a mouse model of neuromyelitis optica.Acta Neuropathol. 2013 Nov;126(5):699-709. doi: 10.1007/s00401-013-1172-z. Epub 2013 Aug 31. Acta Neuropathol. 2013. PMID: 23995423 Free PMC article.
-
Targeting the complement system in neuromyelitis optica spectrum disorder.Expert Opin Biol Ther. 2021 Aug;21(8):1073-1086. doi: 10.1080/14712598.2021.1884223. Epub 2021 Feb 16. Expert Opin Biol Ther. 2021. PMID: 33513036 Free PMC article. Review.
-
Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO.Brain Pathol. 2013 Nov;23(6):684-95. doi: 10.1111/bpa.12085. Brain Pathol. 2013. PMID: 24118484 Free PMC article. Review.
Cited by
-
What's new in neuromyelitis optica spectrum disorder treatment?Taiwan J Ophthalmol. 2022 Sep 1;12(3):249-263. doi: 10.4103/2211-5056.355617. eCollection 2022 Jul-Sep. Taiwan J Ophthalmol. 2022. PMID: 36248092 Free PMC article. Review.
-
A novel aquaporin-4-associated optic neuritis rat model with severe pathological and functional manifestations.J Neuroinflammation. 2022 Oct 27;19(1):263. doi: 10.1186/s12974-022-02623-7. J Neuroinflammation. 2022. PMID: 36303157 Free PMC article.
-
Astrocyte polarization in glaucoma: a new opportunity.Neural Regen Res. 2022 Dec;17(12):2582-2588. doi: 10.4103/1673-5374.339470. Neural Regen Res. 2022. PMID: 35662185 Free PMC article. Review.
-
Hope for patients with neuromyelitis optica spectrum disorders - from mechanisms to trials.Nat Rev Neurol. 2021 Dec;17(12):759-773. doi: 10.1038/s41582-021-00568-8. Epub 2021 Oct 28. Nat Rev Neurol. 2021. PMID: 34711906 Review.
-
The Pathological Activation of Microglia Is Modulated by Sexually Dimorphic Pathways.Int J Mol Sci. 2023 Mar 1;24(5):4739. doi: 10.3390/ijms24054739. Int J Mol Sci. 2023. PMID: 36902168 Free PMC article. Review.
References
-
- Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, Kayagaki N, Yagita H, Okumura K, Imawari M. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol. 1997;158:5283–5291. - PubMed
-
- Avadhanula V, Rodriguez CA, Ulett GC, Bakaletz LO, Adderson EE. Nontypeable haemophilus influenzae adheres to intercellular adhesion molecule 1 (ICAM-1) on respiratory epithelial cells and upregulates ICAM-1 expression. Infect Immun. 2006;74:830–838. doi: 10.1128/IAI.74.2.830-838.2006. - DOI - PMC - PubMed

