Selective deletion of Eos (Ikzf4) in T-regulatory cells leads to loss of suppressive function and development of systemic autoimmunity
- PMID: 31296356
- PMCID: PMC11046398
- DOI: 10.1016/j.jaut.2019.06.011
Selective deletion of Eos (Ikzf4) in T-regulatory cells leads to loss of suppressive function and development of systemic autoimmunity
Erratum in
-
Corrigendum to "Selective deletion of Eos (Ikzf4) in T-regulatory cells leads to loss of suppressive function and development of systemic autoimmunity" [J. Autoimmun. 105C (2019) 102300].J Autoimmun. 2020 Nov;114:102544. doi: 10.1016/j.jaut.2020.102544. Epub 2020 Sep 24. J Autoimmun. 2020. PMID: 32981806 No abstract available.
Abstract
Eos (lkzf4) is a member of the Ikaros family of transcription factors and is preferentially expressed in T-regulatory (Treg) cells. However, the role of Eos in Treg function is controversial. One study using siRNA knock down of Eos demonstrated that it was critical for Treg suppressor function. In contrast, Treg from mice with a global deficiency of Eos had normal Treg function in vitro and in vivo. To further dissect the function of Eos in Tregs, we generated mice with a conditional knock out of Eos in Treg cells (lkzf4fl/fl X Foxp3YFP-cre, Eos cKO). Deletion of Eos in Treg resulted in activation of CD4+Foxp3- and CD8+ T cells at the age of 3 months, cellular infiltration in non-lymphoid tissues, hyperglobulinemia, and anti-nuclear antibodies. While Tregs from Eos cKO mice displayed normal suppressive function in vitro, Eos cKO mice developed severe Experimental Autoimmune Encephalomyletis (EAE) following immunization with myelin oligodendrocyte glycoprotein (MOG) and Eos cKO Treg were unable to suppress Inflammatory Bowel Disease (IBD). Eos cKO mice had decreased growth of the transplantable murine adenocarcinoma MC38 tumor accompanied by enhanced IFN-γ/TNF-α production by CD8+ T cells in tumor draining lymph nodes. Mice with a global deficiency of Eos or a deficiency of Eos only in T cells developed autoimmunity at a much older age (12 months or 7-8 months, respectively). Taken together, Eos appears to play an essential role in multiple aspects of Treg suppressor function, but also plays an as yet unknown role in the function of CD4+Foxp3- and CD8+ T cells and potentially in non-T cells.
Keywords: EAE; Eos; Foxp3; T effector cells; T regulatory cells; Transcription factor.
Published by Elsevier Ltd.
Figures




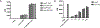


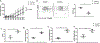
Similar articles
-
Eos Is Redundant for Regulatory T Cell Function but Plays an Important Role in IL-2 and Th17 Production by CD4+ Conventional T Cells.J Immunol. 2015 Jul 15;195(2):553-63. doi: 10.4049/jimmunol.1500627. Epub 2015 Jun 10. J Immunol. 2015. PMID: 26062998 Free PMC article.
-
A GMCSF-Neuroantigen Tolerogenic Vaccine Elicits Systemic Lymphocytosis of CD4+ CD25high FOXP3+ Regulatory T Cells in Myelin-Specific TCR Transgenic Mice Contingent Upon Low-Efficiency T Cell Antigen Receptor Recognition.Front Immunol. 2019 Jan 10;9:3119. doi: 10.3389/fimmu.2018.03119. eCollection 2018. Front Immunol. 2019. PMID: 30687323 Free PMC article.
-
Antigen-specific splenic CD4+ and CD8+ regulatory T cells generated via the eye, suppress experimental autoimmune encephalomyelitis either at the priming or at the effector phase.Int Immunol. 2011 Feb;23(2):119-28. doi: 10.1093/intimm/dxq461. Epub 2011 Jan 27. Int Immunol. 2011. PMID: 21273399 Free PMC article.
-
Interplay between pathogenic Th17 and regulatory T cells.Ann Rheum Dis. 2007 Nov;66 Suppl 3(Suppl 3):iii87-90. doi: 10.1136/ard.2007.078527. Ann Rheum Dis. 2007. PMID: 17934104 Free PMC article. Review.
-
Helios: still behind the clouds.Immunology. 2019 Nov;158(3):161-170. doi: 10.1111/imm.13115. Epub 2019 Oct 13. Immunology. 2019. PMID: 31517385 Free PMC article. Review.
Cited by
-
Inferring upstream regulatory genes of FOXP3 in human regulatory T cells from time-series transcriptomic data.NPJ Syst Biol Appl. 2024 May 29;10(1):59. doi: 10.1038/s41540-024-00387-9. NPJ Syst Biol Appl. 2024. PMID: 38811598 Free PMC article.
-
Foxp3 depends on Ikaros for control of regulatory T cell gene expression and function.Elife. 2024 Apr 24;12:RP91392. doi: 10.7554/eLife.91392. Elife. 2024. PMID: 38655862 Free PMC article.
-
Inhibiting the coregulator CoREST impairs Foxp3+ Treg function and promotes antitumor immunity.J Clin Invest. 2020 Apr 1;130(4):1830-1842. doi: 10.1172/JCI131375. J Clin Invest. 2020. PMID: 31917688 Free PMC article.
-
Non-HLA Gene Polymorphisms in the Pathogenesis of Type 1 Diabetes: Phase and Endotype Specific Effects.Front Immunol. 2022 Jun 21;13:909020. doi: 10.3389/fimmu.2022.909020. eCollection 2022. Front Immunol. 2022. PMID: 35812428 Free PMC article.
-
Long noncoding RNA LIRIL2R modulates FOXP3 levels and suppressive function of human CD4+ regulatory T cells by regulating IL2RA.Proc Natl Acad Sci U S A. 2024 Jun 4;121(23):e2315363121. doi: 10.1073/pnas.2315363121. Epub 2024 May 28. Proc Natl Acad Sci U S A. 2024. PMID: 38805281 Free PMC article.
References
-
- Honma Y, Kiyosawa H, Mori T, Oguri A, Nikaido T, Kanazawa K, et al., Eos: a novel member of the Ikaros gene family expressed predominantly in the developing nervous system, FEBS Lett. 447 (1999) 76–80. - PubMed
-
- Georgopoulos K, Winandy S, Avitahl N, The role of Ikaros in lymphocyte development and homeostasis, Annu. Rev. Immunol. 15 (1997) 155–176. - PubMed
-
- Perdomo J, Holmes M, Chong B, Crossley M, Eos and pegasus, two members of the Ikaros family of proteins with distinct DNA binding activities, J. Biol. Chem. 275 (2000) 38347–383454. - PubMed
-
- Bao J, Lin H, Ouyang Y, Lei D, Osman A, Kim TW, et al., Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos, Nat. Neurosci. 7 (2004) 1250–1258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

