The role of the optic tectum for visually evoked orienting and evasive movements
- PMID: 31296565
- PMCID: PMC6660747
- DOI: 10.1073/pnas.1907962116
The role of the optic tectum for visually evoked orienting and evasive movements
Abstract
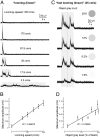
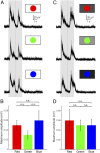
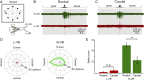
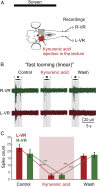
As animals forage for food and water or evade predators, they must rapidly decide what visual features in the environment deserve attention. In vertebrates, this visuomotor computation is implemented within the neural circuits of the optic tectum (superior colliculus in mammals). However, the mechanisms by which tectum decides whether to approach or evade remain unclear, and also which neural mechanisms underlie this behavioral choice. To address this problem, we used an eye-brain-spinal cord preparation to evaluate how the lamprey responds to visual inputs with distinct stimulus-dependent motor patterns. Using ventral root activity as a behavioral readout, we classified 2 main types of fictive motor responses: (i) a unilateral burst response corresponding to orientation of the head toward slowly expanding or moving stimuli, particularly within the anterior visual field, and (ii) a unilateral or bilateral burst response triggering fictive avoidance in response to rapidly expanding looming stimuli or moving bars. A selective pharmacological blockade revealed that the brainstem-projecting neurons in the deep layer of the tectum in interaction with local inhibitory interneurons are responsible for selecting between these 2 visually triggered motor actions conveyed through downstream reticulospinal circuits. We suggest that these visual decision-making circuits had evolved in the common ancestor of vertebrates and have been conserved throughout vertebrate phylogeny.
Keywords: evolution; superior colliculus; visuomotor transformation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Kaas J. H., Topographic maps are fundamental to sensory processing. Brain Res. Bull. 44, 107–112 (1997). - PubMed
-
- Northmore D., “The optic tectum.” The Encyclopedia of Fish Physiology: From Genome to Environment, Farrell A., Ed. (Academic Press, 2011), pp. 131–142.
-
- Butler A. B., Evolution of brains, cognition, and consciousness. Brain Res. Bull. 75, 442–449 (2008). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

