Temporal and differential regulation of KAISO-controlled transcription by phosphorylated and acetylated p53 highlights a crucial regulatory role of apoptosis
- PMID: 31296660
- PMCID: PMC6721929
- DOI: 10.1074/jbc.RA119.008100
Temporal and differential regulation of KAISO-controlled transcription by phosphorylated and acetylated p53 highlights a crucial regulatory role of apoptosis
Abstract
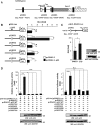
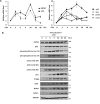
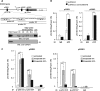
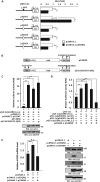
Transcriptional regulator KAISO plays a critical role in cell cycle arrest and apoptosis through modulation of p53 acetylation by histone acetyltransferase p300. KAISO potently stimulates apoptosis in cells expressing WT p53, but not in p53-mutant or p53-null cells. Here, we investigated how KAISO transcription is regulated by p53, finding four potential p53-binding sites (p53-responsive DNA elements; p53REs) located in a distal 5'-upstream regulatory element, intron 1, exon 2 coding sequence, and a 3'-UTR region. Transient transcription assays of pG5-p53RE-Luc constructs with various p53REs revealed that p53 activates KAISO (ZBTB33) transcription by acting on p53RE1 (-4326 to -4227) of the 5'-upstream region and on p53RE3 (+2929 to +2959) of the exon 2 coding region during early DNA damage responses (DDRs). ChIP and oligonucleotide pulldown assays further disclosed that p53 binds to the p53RE1 and p53RE3 sites. Moreover, ataxia telangiectasia mutated (ATM) or ATM-Rad3-related (ATR) kinase-mediated p53 phosphorylation at Ser-15 or Ser-37 residues activated KAISO transcription by binding its p53RE1 or p53RE3 sites during early DDR. p53RE1 uniquely contained three p53-binding half-sites, a structural feature important for transcriptional activation by phosphorylated p53 Ser-15·Ser-37. During the later DDR phase, a KAISO-mediated acetylated p53 form (represented by a p53QRQ acetyl-mimic) robustly activated transcription by acting on p53RE1 in which this structural feature is not significant, but it provided sufficient KAISO levels to confer a p53 "apoptotic code." These results suggest that the critical apoptosis regulator KAISO is a p53 target gene that is differently regulated by phosphorylated p53 or acetylated p53, depending on DDR stage.
Keywords: DNA damage; DNA damage response; DNA damage response (DDR); KAISO; apoptosis; gene transcription; mutant p53; p53; p53QRQ; post-translational modification (PTM); stress response; transcription factor; transcriptional regulation; zinc finger and BTB domain-containing 33 (ZBTB33).
© 2019 Choi et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Daniel J. M., Spring C. M., Crawford H. C., Reynolds A. B., and Baig A. (2002) The p120(ctn)-binding partner Kaiso is a bi-modal DNA-binding protein that recognizes both a sequence-specific consensus and methylated CpG dinucleotides. Nucleic Acids Res. 30, 2911–2919 10.1093/nar/gkf398 - DOI - PMC - PubMed
-
- Lopes E. C., Valls E., Figueroa M. E., Mazur A., Meng F. G., Chiosis G., Laird P. W., Schreiber-Agus N., Greally J. M., Prokhortchouk E., and Melnick A. (2008) Kaiso contributes to DNA methylation-dependent silencing of tumor suppressor genes in colon cancer cell lines. Cancer Res. 68, 7258–7263 10.1158/0008-5472.CAN-08-0344 - DOI - PubMed
-
- Park J. I., Kim S. W., Lyons J. P., Ji H., Nguyen T. T., Cho K., Barton M. C., Deroo T., Vleminckx K., Moon R. T., and McCrea P. D. (2005) Kaiso/p120-catenin and TCF/β-catenin complexes coordinately regulate canonical Wnt gene targets. Dev. Cell 8, 843–854 10.1016/j.devcel.2005.04.010 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

