The missing link: allostery and catalysis in the anti-viral protein SAMHD1
- PMID: 31296733
- PMCID: PMC7045340
- DOI: 10.1042/BST20180348
The missing link: allostery and catalysis in the anti-viral protein SAMHD1
Abstract
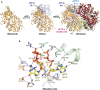
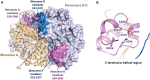
Vertebrate protein SAMHD1 (sterile-α-motif and HD domain containing protein 1) regulates the cellular dNTP (2'-deoxynucleoside-5'-triphosphate) pool by catalysing the hydrolysis of dNTP into 2'-deoxynucleoside and triphosphate products. As an important regulator of cell proliferation and a key player in dNTP homeostasis, mutations to SAMHD1 are implicated in hypermutated cancers, and germline mutations are associated with Chronic Lymphocytic Leukaemia and the inflammatory disorder Aicardi-Goutières Syndrome. By limiting the supply of dNTPs for viral DNA synthesis, SAMHD1 also restricts the replication of several retroviruses, such as HIV-1, and some DNA viruses in dendritic and myeloid lineage cells and resting T-cells. SAMHD1 activity is regulated throughout the cell cycle, both at the level of protein expression and post-translationally, through phosphorylation. In addition, allosteric regulation further fine-tunes the catalytic activity of SAMHD1, with a nucleotide-activated homotetramer as the catalytically active form of the protein. In cells, GTP and dATP are the likely physiological activators of two adjacent allosteric sites, AL1 (GTP) and AL2 (dATP), that bridge monomer-monomer interfaces to stabilise the protein homotetramer. This review summarises the extensive X-ray crystallographic, biophysical and molecular dynamics experiments that have elucidated important features of allosteric regulation in SAMHD1. We present a comprehensive mechanism detailing the structural and protein dynamics components of the allosteric coupling between nucleotide-induced tetramerization and the catalysis of dNTP hydrolysis by SAMHD1.
Keywords: HD domain; HIV; SAMHD1; allosteric regulation; hydrolase; oligomerization.
© 2019 The Author(s).
Conflict of interest statement
The Authors declare that there are no competing interests associated with the manuscript.
Figures
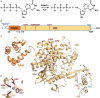


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

