Zinc-binding to the cytoplasmic PAS domain regulates the essential WalK histidine kinase of Staphylococcus aureus
- PMID: 31296851
- PMCID: PMC6624279
- DOI: 10.1038/s41467-019-10932-4
Zinc-binding to the cytoplasmic PAS domain regulates the essential WalK histidine kinase of Staphylococcus aureus
Abstract
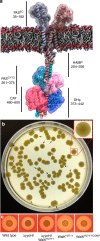
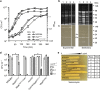
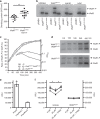
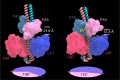
WalKR (YycFG) is the only essential two-component regulator in the human pathogen Staphylococcus aureus. WalKR regulates peptidoglycan synthesis, but this function alone does not explain its essentiality. Here, to further understand WalKR function, we investigate a suppressor mutant that arose when WalKR activity was impaired; a histidine to tyrosine substitution (H271Y) in the cytoplasmic Per-Arnt-Sim (PASCYT) domain of the histidine kinase WalK. Introducing the WalKH271Y mutation into wild-type S. aureus activates the WalKR regulon. Structural analyses of the WalK PASCYT domain reveal a metal-binding site, in which a zinc ion (Zn2+) is tetrahedrally-coordinated by four amino acids including H271. The WalKH271Y mutation abrogates metal binding, increasing WalK kinase activity and WalR phosphorylation. Thus, Zn2+-binding negatively regulates WalKR. Promoter-reporter experiments using S. aureus confirm Zn2+ sensing by this system. Identification of a metal ligand recognized by the WalKR system broadens our understanding of this critical S. aureus regulon.
Conflict of interest statement
The authors declare no competing interests.
Figures

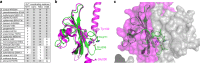
References
-
- Howden BP, Davies JK, Johnson PD, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin. Microbiol. Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. - DOI - PMC - PubMed
-
- Kelley PG, Gao W, Ward PB, Howden BP. Daptomycin non-susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J. Antimicrob. Chemother. 2011;66:1057–1060. doi: 10.1093/jac/dkr066. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GNT1142695/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1136021/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1105905/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1010776/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1044414/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1105525/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1129589/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1145075/Department of Health | National Health and Medical Research Council (NHMRC)/International
- GNT1049192/Department of Health | National Health and Medical Research Council (NHMRC)/International
- DP170102102/Department of Education and Training | Australian Research Council (ARC)/International
- FT170100006/Department of Education and Training | Australian Research Council (ARC)/International
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

