Molecular bilayer graphene
- PMID: 31296875
- PMCID: PMC6624274
- DOI: 10.1038/s41467-019-11098-9
Molecular bilayer graphene
Abstract
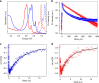
Bilayer graphene consists of two stacked graphene layers bound together by van der Waals interaction. As the molecular analog of bilayer graphene, molecular bilayer graphene (MBLG) can offer useful insights into the structural and functional properties of bilayer graphene. However, synthesis of MBLG, which requires discrete assembly of two graphene fragments, has proved to be challenging. Here, we show the synthesis and characterization of two structurally well-defined MBLGs, both consisting of two π-π stacked nanographene sheets. We find they have excellent stability against variation of concentration, temperature and solvents. The MBLGs show sharp absorption and emission peaks, and further time-resolved spectroscopic studies reveal drastically different lifetimes for the bright and dark Davydov states in these MBLGs.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

