IL-22-binding protein exacerbates influenza, bacterial super-infection
- PMID: 31296910
- PMCID: PMC6717528
- DOI: 10.1038/s41385-019-0188-7
IL-22-binding protein exacerbates influenza, bacterial super-infection
Abstract
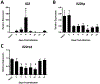
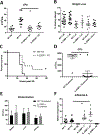
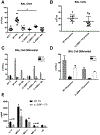
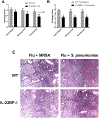
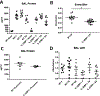
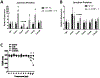
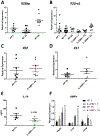
Secondary bacterial pneumonia is a significant complication of severe influenza infection and Staphylococcus aureus and Streptococcus pneumoniae are the primary pathogens of interest. IL-22 promotes S. aureus and S. pneumoniae host defense in the lung through epithelial integrity and induction of antimicrobial peptides and is inhibited by the soluble decoy receptor IL-22-binding protein (IL-22BP). Little is known about the effect of the IL-22/IL-22BP regulatory pathway on lung infection, and it has not been studied in the setting of super-infection. We exposed wild-type and IL-22BP-/- mice to influenza A/PR/8/34 for 6 days prior to infection with S. aureus (USA300) S. pneumoniae. Super-infected IL-22BP-/- mice had decreased bacterial burden and improved survival compared to controls. IL-22BP-/- mice exhibited decreased inflammation, increased lipocalin 2 expression, and deletion of IL-22BP was associated with preserved epithelial barrier function with evidence of improved tight junction stability. Human bronchial epithelial cells treated with IL-22Fc showed evidence of improved tight junctions compared to untreated cells. This study revealed that IL-22BP-/- mice are protected during influenza, bacterial super-infection, suggesting that IL-22BP has a pro-inflammatory role and impairs epithelial barrier function likely through interaction with IL-22.
Conflict of interest statement
Disclosures
The authors have no conflicts of interest to report.
Figures


References
-
- Bacterial coinfections in lung tissue specimens from fatal cases of 2009 pandemic influenza A (H1N1) - United States, May-August 2009. MMWR Morb Mortal Wkly Rep 2009; 58(38): 1071–1074. - PubMed
-
- Finelli L, Fiore A, Dhara R, Brammer L, Shay DK, Kamimoto L et al. Influenza-associated pediatric mortality in the United States: increase of Staphylococcus aureus coinfection. Pediatrics 2008; 122(4): 805–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

