Surgical and tissue engineering strategies for articular cartilage and meniscus repair
- PMID: 31296933
- PMCID: PMC7192556
- DOI: 10.1038/s41584-019-0255-1
Surgical and tissue engineering strategies for articular cartilage and meniscus repair
Abstract
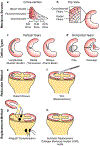
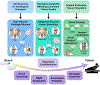
Injuries to articular cartilage and menisci can lead to cartilage degeneration that ultimately results in arthritis. Different forms of arthritis affect ~50 million people in the USA alone, and it is therefore crucial to identify methods that will halt or slow the progression to arthritis, starting with the initiating events of cartilage and meniscus defects. The surgical approaches in current use have a limited capacity for tissue regeneration and yield only short-term relief of symptoms. Tissue engineering approaches are emerging as alternatives to current surgical methods for cartilage and meniscus repair. Several cell-based and tissue-engineered products are currently in clinical trials for cartilage lesions and meniscal tears, opening new avenues for cartilage and meniscus regeneration. This Review provides a summary of surgical techniques, including tissue-engineered products, that are currently in clinical use, as well as a discussion of state-of-the-art tissue engineering strategies and technologies that are being developed for use in articular cartilage and meniscus repair and regeneration. The obstacles to clinical translation of these strategies are also included to inform the development of innovative tissue engineering approaches.
Conflict of interest statement
Competing interests
W.E.B. declares she is the Director of Outreach and a social media contributor for Science Cheerleaders, Incorporated. C.A.L. declares she is on the advisory board of Vericel. N.P. declares he is an associate editor of the Arthroscopy Journal. K.A.A. declares he is on the scientific advisory board of Histogenics. K.A.A., J.C.H., H.K. and W.E.B. declare they are listed as co-authors of submitted US patent applications (16/136,894 and 16/137,120). D.A. declares no competing interests.
Figures

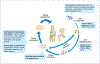
References
-
- Centers for Disease Control and Prevention. Arthritis-related statistics, <https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm> (2018).
-
- Centers for Disease Control and Prevention. Osteoarthritis (OA), <https://www.cdc.gov/arthritis/basics/osteoarthritis.htm> (2019).
-
- Wilder FV, Hall BJ, Barrett JP Jr. & Lemrow NB History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The Clearwater Osteoarthritis Study. Osteoarthritis Cartilage 10, 611–616 (2002). - PubMed

