Early or deferred treatment of smoldering multiple myeloma: a meta-analysis on randomized controlled studies
- PMID: 31296995
- PMCID: PMC6595476
- DOI: 10.2147/CMAR.S205623
Early or deferred treatment of smoldering multiple myeloma: a meta-analysis on randomized controlled studies
Abstract
Purpose: Smoldering multiple myeloma (SMM) is a rare asymptomatic plasma cell disorder. Even with emerging therapeutic approaches and risk stratification, the optimal time to treat SMM remains controversial. This meta-analysis aimed to compare early treatment with deferred treatment of SMM, especially high-risk SMM.
Methods: Early treatment was defined as treatment immediately after diagnosis. Deferred treatment was initiated after progression. The primary outcome was progression. Secondary outcomes were mortality, response, and safety. PubMed, EMBASE, Medline, Cochrane, and ClinicalTrials.gov databases were searched from January 1990 to March 2019. Randomized controlled trials (RCTs) comparing early treatment with deferred treatment in SMM patients were eligible. Risk ratios (RRs) with 95% confidence interval (CI) were pooled.
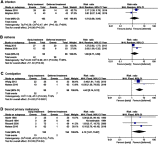
Results: Eight RCTs covering 885 SMM patients were included. Considering all the different treatment approaches, early treatment significantly decreased progression of SMM (RR=0.53, 95% CI 0.33-0.87, P=0.01). In subgroup analysis, melphalan plus prednisone (RR=0.22, 95% CI 0.08-0.64, P=0.005) and immuno-modulatory drugs (RR=0.43, 95% CI 0.31-0.59, P<0.00001) significantly reduced progression. However, neither mortality nor response rate was significantly affected by early treatment. In terms of high-risk SMM patients, early treatment significantly decreased both progression (RR=0.51, 95% CI 0.37-0.70, P=0.0001) and mortality (RR=0.53, 95% CI 0.29-0.96, P=0.04). Frequently seen adverse events were infection, constipation, asthenia, and second primary malignancy. A remarkably elevated risk of constipation was associated with early treatment using immuno-modulatory agents (RR=4.43, 95% CI 2.14-9.12, P<0.0001). Second primary malignancy was significantly increased with early treatment (RR=4.13, 95% CI 1.07-15.97, P=0.04). No significant difference was identified in infection or asthenia.
Conclusion: These findings suggest that early treatment could decrease progression and mortality of high-risk SMM patients with a tolerable safety profile.
Keywords: early treatment; lenalidomide; meta-analysis; smoldering multiple myeloma.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures







Similar articles
-
Early versus deferred treatment for smoldering multiple myeloma: a meta-analysis of randomized, controlled trials.PLoS One. 2014 Oct 3;9(10):e109758. doi: 10.1371/journal.pone.0109758. eCollection 2014. PLoS One. 2014. PMID: 25279718 Free PMC article.
-
Observation or treatment for smoldering multiple myeloma? A systematic review and meta-analysis of randomized controlled studies.Blood Cancer J. 2025 May 26;15(1):104. doi: 10.1038/s41408-025-01312-x. Blood Cancer J. 2025. PMID: 40419473 Free PMC article.
-
Immediate versus deferred percutaneous coronary intervention for patients with acute coronary syndrome: A meta-analysis of randomized controlled trials.PLoS One. 2020 Jul 2;15(7):e0234655. doi: 10.1371/journal.pone.0234655. eCollection 2020. PLoS One. 2020. PMID: 32614851 Free PMC article.
-
Therapeutic Advances in the Management of Smoldering Myeloma.Am J Ther. 2020 Mar/Apr;27(2):e194-e203. doi: 10.1097/MJT.0000000000001034. Am J Ther. 2020. PMID: 31842112 Review.
-
Progress in the Management of Smoldering Multiple Myeloma.Curr Hematol Malig Rep. 2021 Apr;16(2):172-182. doi: 10.1007/s11899-021-00623-7. Epub 2021 May 13. Curr Hematol Malig Rep. 2021. PMID: 33983517 Review.
Cited by
-
Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies.Blood Cancer J. 2021 May 17;11(5):94. doi: 10.1038/s41408-021-00480-w. Blood Cancer J. 2021. PMID: 34001889 Free PMC article. Clinical Trial.
-
The impact of lag time to cancer diagnosis and treatment on clinical outcomes prior to the COVID-19 pandemic: A scoping review of systematic reviews and meta-analyses.Elife. 2023 Jan 31;12:e81354. doi: 10.7554/eLife.81354. Elife. 2023. PMID: 36718985 Free PMC article.
-
2021 European Myeloma Network review and consensus statement on smoldering multiple myeloma: how to distinguish (and manage) Dr. Jekyll and Mr. Hyde.Haematologica. 2021 Nov 1;106(11):2799-2812. doi: 10.3324/haematol.2021.278519. Haematologica. 2021. PMID: 34261295 Free PMC article. Review.
-
Association between low-income subsidies and inequities in orally administered antimyeloma therapy use.Am J Manag Care. 2023 May;29(5):246-254. doi: 10.37765/ajmc.2023.89357. Am J Manag Care. 2023. PMID: 37229783 Free PMC article.
-
What Is New in the Treatment of Smoldering Multiple Myeloma?J Clin Med. 2021 Jan 22;10(3):421. doi: 10.3390/jcm10030421. J Clin Med. 2021. PMID: 33499196 Free PMC article. Review.
References
-
- Muchtar E, Kumar SK, Magen H, Gertz MA. Diagnosis and management of smoldering multiple myeloma: the razor’s edge between clonality and cancer. Leuk Lymphoma. 2018;59(2):288–299. - PubMed
LinkOut - more resources
Full Text Sources

