The Post-amyloid Era in Alzheimer's Disease: Trust Your Gut Feeling
- PMID: 31297054
- PMCID: PMC6608545
- DOI: 10.3389/fnagi.2019.00143
The Post-amyloid Era in Alzheimer's Disease: Trust Your Gut Feeling
Abstract
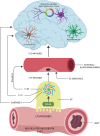
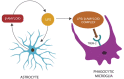
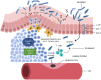
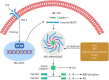
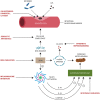
The amyloid hypothesis, the assumption that beta-amyloid toxicity is the primary cause of neuronal and synaptic loss, has been the mainstream research concept in Alzheimer's disease for the past two decades. Currently, this model is quietly being replaced by a more holistic, "systemic disease" paradigm which, like the aging process, affects multiple body tissues and organs, including the gut microbiota. It is well-established that inflammation is a hallmark of cellular senescence; however, the infection-senescence link has been less explored. Microbiota-induced senescence is a gradually emerging concept promoted by the discovery of pathogens and their products in Alzheimer's disease brains associated with senescent neurons, glia, and endothelial cells. Infectious agents have previously been associated with Alzheimer's disease, but the cause vs. effect issue could not be resolved. A recent study may have settled this debate as it shows that gingipain, a Porphyromonas gingivalis toxin, can be detected not only in Alzheimer's disease but also in the brains of older individuals deceased prior to developing the illness. In this review, we take the position that gut and other microbes from the body periphery reach the brain by triggering intestinal and blood-brain barrier senescence and disruption. We also surmise that novel Alzheimer's disease findings, including neuronal somatic mosaicism, iron dyshomeostasis, aggressive glial phenotypes, and loss of aerobic glycolysis, can be explained by the infection-senescence model. In addition, we discuss potential cellular senescence targets and therapeutic strategies, including iron chelators, inflammasome inhibitors, senolytic antibiotics, mitophagy inducers, and epigenetic metabolic reprograming.
Keywords: amyloid hypothesis; infection; inflammation; microbiome; senescence.
Figures
Similar articles
-
Time to test antibacterial therapy in Alzheimer's disease.Brain. 2019 Oct 1;142(10):2905-2929. doi: 10.1093/brain/awz244. Brain. 2019. PMID: 31532495 Review.
-
Effects of chronic noise exposure on the microbiome-gut-brain axis in senescence-accelerated prone mice: implications for Alzheimer's disease.J Neuroinflammation. 2018 Jun 22;15(1):190. doi: 10.1186/s12974-018-1223-4. J Neuroinflammation. 2018. PMID: 29933742 Free PMC article.
-
Astrocyte Senescence and Alzheimer's Disease: A Review.Front Aging Neurosci. 2020 Jun 9;12:148. doi: 10.3389/fnagi.2020.00148. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32581763 Free PMC article. Review.
-
The relationship between iron dyshomeostasis and amyloidogenesis in Alzheimer's disease: Two sides of the same coin.Neurobiol Dis. 2015 Sep;81:49-65. doi: 10.1016/j.nbd.2015.08.007. Epub 2015 Aug 22. Neurobiol Dis. 2015. PMID: 26303889 Free PMC article. Review.
-
Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer's disease animal model.Gut. 2020 Feb;69(2):283-294. doi: 10.1136/gutjnl-2018-317431. Epub 2019 Aug 30. Gut. 2020. PMID: 31471351
Cited by
-
Altered Mitochondrial Morphology and Bioenergetics in a New Yeast Model Expressing Aβ42.Int J Mol Sci. 2023 Jan 4;24(2):900. doi: 10.3390/ijms24020900. Int J Mol Sci. 2023. PMID: 36674415 Free PMC article.
-
A New Integrative Theory of Brain-Body-Ecosystem Medicine: From the Hippocratic Holistic View of Medicine to Our Modern Society.Int J Environ Res Public Health. 2019 Aug 28;16(17):3136. doi: 10.3390/ijerph16173136. Int J Environ Res Public Health. 2019. PMID: 31466374 Free PMC article.
-
Potential therapeutic effects of boswellic acids/Boswellia serrata extract in the prevention and therapy of type 2 diabetes and Alzheimer's disease.Naunyn Schmiedebergs Arch Pharmacol. 2021 Nov;394(11):2167-2185. doi: 10.1007/s00210-021-02154-7. Epub 2021 Sep 20. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 34542667 Review.
-
Infection threat shapes our social instincts.Behav Ecol Sociobiol. 2021;75(3):47. doi: 10.1007/s00265-021-02975-9. Epub 2021 Feb 10. Behav Ecol Sociobiol. 2021. PMID: 33583997 Free PMC article. Review.
-
Endothelial Senescence and Chronic Fatigue Syndrome, a COVID-19 Based Hypothesis.Front Cell Neurosci. 2021 Jun 25;15:673217. doi: 10.3389/fncel.2021.673217. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34248502 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

