Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16
- PMID: 31297151
- PMCID: PMC6598341
- DOI: 10.1186/s13068-019-1512-x
Efficient biochemical production of acetoin from carbon dioxide using Cupriavidus necator H16
Abstract
Background: Cupriavidus necator is the best-studied knallgas (also termed hydrogen oxidizing) bacterium and provides a model organism for studying the production of the storage polymer polyhydroxybutyrate (PHB). Genetically engineered strains could be applied for the autotrophic production of valuable chemicals. Nevertheless, the efficiency of the catalyzed processes is generally believed to be lower than with acetogenic bacteria. Experimental data on the potential efficiency of autotrophic production with C. necator are sparse. Hence, this study aimed at developing a strain for the production of the bulk chemical acetoin from carbon dioxide and to analyze the carbon and electron yield in detail.
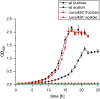
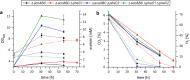
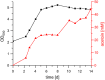
Results: We developed a constitutive promoter system based on the natural PHB promoter of this organism. Codon-optimized versions of the acetolactate dehydrogenase (alsS) and acetolactate decarboxylase (alsD) from Bacillus subtilis were cloned under control of the PHB promoter in order to produce acetoin from pyruvate. The production process's efficiency could be significantly increased by deleting the PHB synthase phaC2. Further deletion of the other PHB synthase encoded in the genome (phaC1) led to a strain that produced acetoin with > 100% carbon efficiency. This increase in efficiency is most probably due to a minor amount of cell lysis. Using a variation in hydrogen and oxygen gas mixtures, we observed that the optimal oxygen concentration for the process was between 15 and 20%.
Conclusion: To the best of our knowledge, this study describes for the first time a highly efficient process for the chemolithoautotrophic production of the platform chemical acetoin.
Keywords: Acetoin; Autotroph; Cupriavidus necator H16; Platform chemical; Polyhydroxybutyrate; Ralstonia eutropha H16.
Conflict of interest statement
Competing interestsThe authors declare that they have no competing interests.
Figures




References
LinkOut - more resources
Full Text Sources

