The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus
- PMID: 31297186
- PMCID: PMC6600864
- DOI: 10.12688/f1000research.18356.2
The neurobiological mechanism underlying hypothalamic GnRH pulse generation: the role of kisspeptin neurons in the arcuate nucleus
Abstract
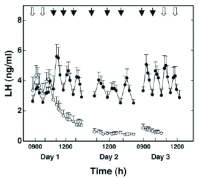
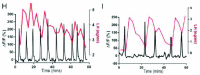
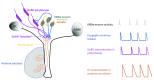
This review recounts the origins and development of the concept of the hypothalamic gonadotropin-releasing hormone (GnRH) pulse generator. It starts in the late 1960s when striking rhythmic episodes of luteinizing hormone secretion, as reflected by circulating concentrations of this gonadotropin, were first observed in monkeys and ends in the present day. It is currently an exciting time witnessing the application, primarily to the mouse, of contemporary neurobiological approaches to delineate the mechanisms whereby Kiss1/NKB/Dyn (KNDy) neurons in the arcuate nucleus of the hypothalamus generate and time the pulsatile output of kisspeptin from their terminals in the median eminence that in turn dictates intermittent GnRH release and entry of this decapeptide into the primary plexus of the hypophysial portal circulation. The review concludes with an examination of questions that remain to be addressed.
Keywords: GnRH; Kisspeptin; dynorphin; neurokinin B; pulse generation.
Conflict of interest statement
No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures
Similar articles
-
Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes.Endocrinology. 2013 Nov;154(11):4259-69. doi: 10.1210/en.2013-1331. Epub 2013 Aug 19. Endocrinology. 2013. PMID: 23959940 Free PMC article.
-
Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse.J Neurosci. 2009 Sep 23;29(38):11859-66. doi: 10.1523/JNEUROSCI.1569-09.2009. J Neurosci. 2009. PMID: 19776272 Free PMC article.
-
Regulation of GnRH pulsatility in ewes.Reproduction. 2018 Sep;156(3):R83-R99. doi: 10.1530/REP-18-0127. Epub 2018 Jun 7. Reproduction. 2018. PMID: 29880718 Free PMC article. Review.
-
The Origin of GnRH Pulse Generation: An Integrative Mathematical-Experimental Approach.J Neurosci. 2019 Dec 4;39(49):9738-9747. doi: 10.1523/JNEUROSCI.0828-19.2019. Epub 2019 Oct 23. J Neurosci. 2019. PMID: 31645462 Free PMC article.
-
Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals.Front Neuroendocrinol. 2022 Jan;64:100968. doi: 10.1016/j.yfrne.2021.100968. Epub 2021 Nov 19. Front Neuroendocrinol. 2022. PMID: 34808231 Review.
Cited by
-
The role of MicroRNAs as fine-tuners in the onset of puberty: a comprehensive review.Ann Pediatr Endocrinol Metab. 2024 Aug;29(4):211-219. doi: 10.6065/apem.2346238.119. Epub 2024 Aug 31. Ann Pediatr Endocrinol Metab. 2024. PMID: 39231482 Free PMC article.
-
Estrogen Regulation of the Molecular Phenotype and Active Translatome of AVPV Kisspeptin Neurons.Endocrinology. 2021 Sep 1;162(9):bqab080. doi: 10.1210/endocr/bqab080. Endocrinology. 2021. PMID: 33856454 Free PMC article.
-
Characterization of the Action of Tachykinin Signaling on Pulsatile LH Secretion in Male Mice.Endocrinology. 2021 Aug 1;162(8):bqab074. doi: 10.1210/endocr/bqab074. Endocrinology. 2021. PMID: 33839770 Free PMC article.
-
Disruptions in Hypothalamic-Pituitary-Gonadal Axis Development and Their IgG Modulation after Prenatal Systemic Inflammation in Male Rats.Int J Mol Sci. 2023 Feb 1;24(3):2726. doi: 10.3390/ijms24032726. Int J Mol Sci. 2023. PMID: 36769048 Free PMC article.
-
Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome.Endocr Rev. 2020 Jul 1;41(4):bnaa010. doi: 10.1210/endrev/bnaa010. Endocr Rev. 2020. PMID: 32310267 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

