Lactate and cancer: a "lactatic" perspective on spinal tumor metabolism (part 1)
- PMID: 31297385
- PMCID: PMC6595211
- DOI: 10.21037/atm.2019.02.32
Lactate and cancer: a "lactatic" perspective on spinal tumor metabolism (part 1)
Abstract
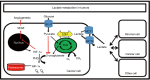
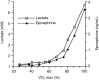
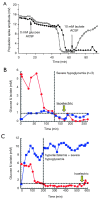
Spine tumors are among the most difficult tumors to treat given their proximity to the spinal cord. Despite advances in adjuvant therapies, surgery remains a critical component of treatment, both in primary tumors and metastatic disease. Given the significant morbidity of these surgeries and with other current adjuvant therapies (e.g., radiation, chemotherapy), interest has grown in other methods of targeting tumors of the spine. Recent efforts have highlighted the tumor microenvironment, and specifically lactate, as central to tumorigenesis. Once erroneously considered a waste product that indicated hypoxia/hypoperfusion, lactate is now known to be at the center of whole-body metabolism, shuttling between tissues and being used as a fuel. Diffusion-driven transporters and the near-equilibrium enzyme lactate dehydrogenase (LDH) allow rapid mobilization of large stores of muscle glycogen in the form of lactate. In times of stress, catecholamines can bind muscle cell receptors and trigger the breakdown of glycogen to lactate, which can then diffuse out into circulation and be used as a fuel where needed. Hypoxia, in contrast, is rarely the reason for an elevated arterial [lactate]. Tumors were originally described in the 1920's as being "glucose-avid" and "lactate-producing" even in normoxia (the "Warburg effect"). We now know that a broad range of metabolic behaviors likely exist, including cancer cells that consume lactate as a fuel, others that may produce it, and still others that may change their behavior based on the local microenvironment. In this review we will examine the relationship between lactate and tumor metabolism with a brief look at spine-specific tumors. Lactate is a valuable fuel and potent signaling molecule that has now been implicated in multiple steps in tumorigenesis [e.g., driving vascular endothelial growth factor (VEGF) expression in normoxia]. Future work should utilize translational animal models to target tumors by altering the local tumor microenvironment, of which lactate is a critical part.
Keywords: Lactate; cancer; lactic acid; neoplasm metastasis; neoplasms; neurosurgery; spinal; spine; tumor.
Conflict of interest statement
Conflicts of Interest: ML Goodwin: Consultant for ROM3, Augmedics; DM Sciubba: Consultant for Orthofix, Globus, K2M, Medtronic, Stryker, Baxter. The other authors have no conflicts of interest to declare.
Figures


Comment in
-
Lactate as a key metabolic intermediate in cancer.Ann Transl Med. 2019 May;7(10):210. doi: 10.21037/atm.2019.01.60. Ann Transl Med. 2019. PMID: 31302666 Free PMC article. No abstract available.
Similar articles
-
Lactate-Protected Hypoglycemia (LPH).Front Neurosci. 2020 Sep 3;14:920. doi: 10.3389/fnins.2020.00920. eCollection 2020. Front Neurosci. 2020. PMID: 33013305 Free PMC article.
-
A lactatic perspective on metabolism.Med Sci Sports Exerc. 2008 Mar;40(3):477-85. doi: 10.1249/MSS.0b013e31815fa580. Med Sci Sports Exerc. 2008. PMID: 18379210 Review.
-
Lactate Dehydrogenases as Metabolic Links between Tumor and Stroma in the Tumor Microenvironment.Cancers (Basel). 2019 May 29;11(6):750. doi: 10.3390/cancers11060750. Cancers (Basel). 2019. PMID: 31146503 Free PMC article. Review.
-
Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy.Front Oncol. 2020 Mar 18;10:231. doi: 10.3389/fonc.2020.00231. eCollection 2020. Front Oncol. 2020. PMID: 32257942 Free PMC article. Review.
-
A microscale mathematical model for metabolic symbiosis: Investigating the effects of metabolic inhibition on ATP turnover in tumors.J Theor Biol. 2015 Feb 7;366:103-14. doi: 10.1016/j.jtbi.2014.11.016. Epub 2014 Nov 27. J Theor Biol. 2015. PMID: 25433213
Cited by
-
A novel lactate metabolism-related signature predicts prognosis and tumor immune microenvironment of breast cancer.Front Genet. 2022 Sep 7;13:934830. doi: 10.3389/fgene.2022.934830. eCollection 2022. Front Genet. 2022. PMID: 36171887 Free PMC article.
-
Mass spectrometric investigations of caloric restriction mimetics.Proteomics. 2021 May;21(9):e2000121. doi: 10.1002/pmic.202000121. Epub 2021 Feb 23. Proteomics. 2021. PMID: 33460282 Free PMC article. Review.
-
Lactate shuttle: from substance exchange to regulatory mechanism.Hum Cell. 2022 Jan;35(1):1-14. doi: 10.1007/s13577-021-00622-z. Epub 2021 Oct 4. Hum Cell. 2022. PMID: 34606041 Review.
-
Mitochondrial lactate metabolism: history and implications for exercise and disease.J Physiol. 2021 Feb;599(3):863-888. doi: 10.1113/JP278930. Epub 2020 May 27. J Physiol. 2021. PMID: 32358865 Free PMC article. Review.
-
Lactate as a key metabolic intermediate in cancer.Ann Transl Med. 2019 May;7(10):210. doi: 10.21037/atm.2019.01.60. Ann Transl Med. 2019. PMID: 31302666 Free PMC article. No abstract available.
References
-
- Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res 1980;153:106-20. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
