Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy
- PMID: 31297795
- PMCID: PMC6687533
- DOI: 10.1111/epi.16283
Identifying the neural basis of a language-impaired phenotype of temporal lobe epilepsy
Abstract
Objective: To identify neuroimaging and clinical biomarkers associated with a language-impaired phenotype in refractory temporal lobe epilepsy (TLE).
Methods: Eighty-five patients with TLE were characterized as language-impaired (TLE-LI) or non-language-impaired (TLE-NLI) based on comprehensive neuropsychological testing. Structural magnetic resonance imaging (MRI), diffusion tensor imaging, and functional MRI (fMRI) were obtained in patients and 47 healthy controls (HC). fMRI activations and cortical thickness were calculated within language regions of interest, and fractional anisotropy (FA) was calculated within deep white matter tracts associated with language. Analyses of variance were performed to test for differences among the groups in imaging measures. Receiver operator characteristic curves were used to determine how well different clinical versus imaging measures discriminated TLE-LI from TLE-NLI.
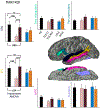
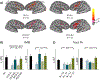
Results: TLE-LI patients showed significantly less activation within left superior temporal cortex compared to HC and TLE-NLI, regardless of side of seizure onset. TLE-LI also showed decreased FA in the inferior longitudinal fasciculus and arcuate fasciculus compared to HC. Cortical thickness did not differ between groups in any region. A model that included language-related fMRI activations within the superior temporal gyrus, age at onset, and demographic variables was the most predictive of language impairment (area under the curve = 0.80).
Significance: These findings demonstrate a unique imaging signature associated with a language-impaired phenotype in TLE, characterized by functional and microstructural alterations within the language network. Reduced left superior temporal activation combined with compromise to language association tracts underlies this phenotype, extending our previous work on cognitive phenotypes that could have implications for treatment-planning or cognitive progression in TLE.
Keywords: clinical biomarkers; cognitive phenotype; diffusion tensor imaging; functional magnetic resonance imaging; neural substrate; neuroimaging.
Wiley Periodicals, Inc. © 2019 International League Against Epilepsy.
Conflict of interest statement
Disclosure
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures


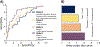
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

