Combining Prospective Acquisition CorrEction (PACE) with retrospective correction to reduce motion artifacts in resting state fMRI data
- PMID: 31297966
- PMCID: PMC6710196
- DOI: 10.1002/brb3.1341
Combining Prospective Acquisition CorrEction (PACE) with retrospective correction to reduce motion artifacts in resting state fMRI data
Abstract
Background: Head movement in the scanner causes spurious signal changes in the blood-oxygen-level-dependent (BOLD) signal, confounding resting state functional connectivity (RSFC) estimates obtained from functional magnetic resonance imaging (fMRI). We examined the effectiveness of Prospective Acquisition CorrEction (PACE) in reducing motion artifacts in BOLD data.
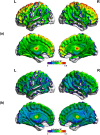
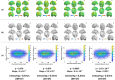
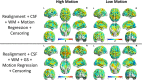
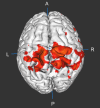
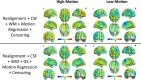
Methods: Using PACE-corrected RS-fMRI data obtained from 44 subjects and subdividing them into low- and high-motion cohorts, we investigated voxel-wise motion-BOLD relationships, the distance-dependent functional connectivity artifact and the correlation between head motion and connectivity metrics such as posterior cingulate seed-based connectivity and network degree centrality.
Results: Our results indicate that, when PACE is used in combination with standard retrospective motion correction strategies, it provides two principal advantages over conventional echo-planar imaging (EPI) RS-fMRI data: (a) PACE was effective in eliminating significant negative motion-BOLD relationships, shown to be associated with signal dropouts caused by head motion, and (b) Censoring with a lower threshold (framewise displacement >0.5 mm) and a smaller window around the motion corrupted time point provided qualitatively equivalent reductions in the motion artifact with PACE when compared to a more conservative threshold of 0.2 mm required with conventional EPI data.
Conclusions: PACE when used in conjunction with retrospective motion correction methods including nuisance signal and motion parameter regression, and censoring, did prove effective in almost eliminating head motion artifacts, even with a lower censoring threshold. Use of a lower censoring threshold could provide substantial savings in data that would otherwise be lost to censoring. Three-dimensional PACE has negligible overhead in terms of scan time, sequence modifications or additional and hence presents an attractive option for head motion correction in high-throughput resting-state BOLD imaging.
Keywords: functional connectivity; head motion; motion artifacts; prospective motion correction; resting state fMRI.
© 2019 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures








Similar articles
-
An evaluation of the efficacy, reliability, and sensitivity of motion correction strategies for resting-state functional MRI.Neuroimage. 2018 May 1;171:415-436. doi: 10.1016/j.neuroimage.2017.12.073. Epub 2017 Dec 24. Neuroimage. 2018. PMID: 29278773
-
Resting state fMRI data from subjects scanned with the EPI-PACE (Echoplanar Imaging - Prospective Acquisition CorrEction) sequence.Data Brief. 2018 Feb 6;20:2072-2075. doi: 10.1016/j.dib.2018.01.089. eCollection 2018 Oct. Data Brief. 2018. PMID: 30345338 Free PMC article.
-
Evaluating the effects of volume censoring on fetal functional connectivity.Sci Rep. 2025 Apr 16;15(1):13181. doi: 10.1038/s41598-025-96538-x. Sci Rep. 2025. PMID: 40240427 Free PMC article.
-
Head Motion and Correction Methods in Resting-state Functional MRI.Magn Reson Med Sci. 2016;15(2):178-86. doi: 10.2463/mrms.rev.2015-0060. Epub 2015 Dec 22. Magn Reson Med Sci. 2016. PMID: 26701695 Free PMC article. Review.
-
Recent progress and outstanding issues in motion correction in resting state fMRI.Neuroimage. 2015 Jan 15;105:536-51. doi: 10.1016/j.neuroimage.2014.10.044. Epub 2014 Oct 24. Neuroimage. 2015. PMID: 25462692 Free PMC article. Review.
Cited by
-
The association between head motion during functional magnetic resonance imaging and executive functioning in older adults.Neuroimage Rep. 2022 Jun;2(2):100085. doi: 10.1016/j.ynirp.2022.100085. Epub 2022 Feb 25. Neuroimage Rep. 2022. PMID: 37377763 Free PMC article.
-
Time of day dependent longitudinal changes in resting-state fMRI.Front Neurol. 2023 Jul 5;14:1166200. doi: 10.3389/fneur.2023.1166200. eCollection 2023. Front Neurol. 2023. PMID: 37475742 Free PMC article.
-
Exploring brain connectivity changes in major depressive disorder using functional-structural data fusion: A CAN-BIND-1 study.Hum Brain Mapp. 2021 Oct 15;42(15):4940-4957. doi: 10.1002/hbm.25590. Epub 2021 Jul 23. Hum Brain Mapp. 2021. PMID: 34296501 Free PMC article.
-
Amygdala activity related to perceived social support.Sci Rep. 2020 Feb 19;10(1):2951. doi: 10.1038/s41598-020-59758-x. Sci Rep. 2020. PMID: 32076036 Free PMC article.
-
Abnormal global signal topography of self modulates emotion dysregulation in major depressive disorder.Transl Psychiatry. 2023 Apr 3;13(1):107. doi: 10.1038/s41398-023-02398-2. Transl Psychiatry. 2023. PMID: 37012231 Free PMC article.
References
-
- Beall, E. B. , & Lowe, M. J. (2014). SimPACE: Generating simulated motion corrupted BOLD data with synthetic‐navigated acquisition for the development and evaluation of SLOMOCO: A new, highly effective slicewise motion correction. NeuroImage, 101, 21–34. 10.1016/j.neuroimage.2014.06.038 - DOI - PMC - PubMed
-
- Coyne, K. (2012). MRI: A guided tour. Retrieved from https://nationalmaglab.org/education/magnet-academy/learn-the-basics/sto.... May 19, 2019.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

