Design, Synthesis, and Characterization of Ogerin-Based Positive Allosteric Modulators for G Protein-Coupled Receptor 68 (GPR68)
- PMID: 31298539
- PMCID: PMC6923801
- DOI: 10.1021/acs.jmedchem.9b00869
Design, Synthesis, and Characterization of Ogerin-Based Positive Allosteric Modulators for G Protein-Coupled Receptor 68 (GPR68)
Abstract
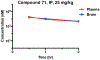
G protein-coupled receptor 68 (GPR68) is an understudied orphan G protein-coupled receptor (GPCR). It is expressed most abundantly in the brain, potentially playing important roles in learning and memory. Pharmacological studies with GPR68 have been hindered by lack of chemical tools that can selectively modulate its activity. We previously reported the first small-molecule positive allosteric modulator (PAM), ogerin (1), and showed that 1 can potentiate proton activity at the GPR68-Gs pathway. Here, we report the first comprehensive structure-activity relationship (SAR) study on the scaffold of 1. Our lead compound resulted from this study, MS48107 (71), displayed 33-fold increased allosteric activity compared to 1. Compound 71 demonstrated high selectivity over closely related proton GPCRs and 48 common drug targets, and was bioavailable and brain-penetrant in mice. Thus, our SAR study has resulted in an improved GPR68 PAM for investigating the physiological and pathophysiological roles of GPR68 in vitro and in vivo.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
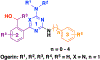




Similar articles
-
Differential Roles of Extracellular Histidine Residues of GPR68 for Proton-Sensing and Allosteric Modulation by Divalent Metal Ions.Biochemistry. 2020 Sep 29;59(38):3594-3614. doi: 10.1021/acs.biochem.0c00576. Epub 2020 Sep 10. Biochemistry. 2020. PMID: 32865988 Free PMC article.
-
Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65.Nature. 2015 Nov 26;527(7579):477-83. doi: 10.1038/nature15699. Epub 2015 Nov 9. Nature. 2015. PMID: 26550826 Free PMC article.
-
Species-Dependent Enhancement of Ovarian Cancer G Protein-Coupled Receptor 1 Activation by Ogerin.Zoolog Sci. 2020 Apr;37(2):103-108. doi: 10.2108/zs190106. Zoolog Sci. 2020. PMID: 32282140
-
GPR68: An Emerging Drug Target in Cancer.Int J Mol Sci. 2019 Jan 28;20(3):559. doi: 10.3390/ijms20030559. Int J Mol Sci. 2019. PMID: 30696114 Free PMC article. Review.
-
Novel Allosteric Modulators of G Protein-coupled Receptors.J Biol Chem. 2015 Aug 7;290(32):19478-88. doi: 10.1074/jbc.R115.662759. Epub 2015 Jun 22. J Biol Chem. 2015. PMID: 26100627 Free PMC article. Review.
Cited by
-
Proton-Sensing GPCRs in Health and Disease.Cells. 2021 Aug 10;10(8):2050. doi: 10.3390/cells10082050. Cells. 2021. PMID: 34440817 Free PMC article. Review.
-
Unbalancing cAMP and Ras/MAPK pathways as a therapeutic strategy for cutaneous neurofibromas.JCI Insight. 2024 Jan 4;9(3):e168826. doi: 10.1172/jci.insight.168826. JCI Insight. 2024. PMID: 38175707 Free PMC article.
-
Proton Sensing GPCR's: The missing link to Warburg's Oncogenic Legacy?J Cancer Biol. 2024;5(2):65-75. doi: 10.46439/cancerbiology.5.066. J Cancer Biol. 2024. PMID: 39641117 Free PMC article.
-
GPR68-ATF4 signaling is a novel prosurvival pathway in glioblastoma activated by acidic extracellular microenvironment.Exp Hematol Oncol. 2024 Jan 31;13(1):13. doi: 10.1186/s40164-023-00468-1. Exp Hematol Oncol. 2024. PMID: 38291540 Free PMC article.
-
Differential Roles of Extracellular Histidine Residues of GPR68 for Proton-Sensing and Allosteric Modulation by Divalent Metal Ions.Biochemistry. 2020 Sep 29;59(38):3594-3614. doi: 10.1021/acs.biochem.0c00576. Epub 2020 Sep 10. Biochemistry. 2020. PMID: 32865988 Free PMC article.
References
-
- Xu Y; Casey G Identification of Human OGR1, a Novel G Protein-Coupled Receptor That Maps to Chromosome 14. Genomics 1996, 35, 397–402. - PubMed
-
- Ludwig M-G; Vanek M; Guerini D; Gasser JA; Jones CE; Junker U; Hofstetter H; Wolf RM; Seuwen K Proton-Sensing G-Protein-Coupled Receptors. Nature 2003, 425, 93–98. - PubMed
-
- Mogi C; Tomura H; Tobo M; Wang J-Q; Damirin A; Kon J; Komachi M; Hashimoto K; Sato K; Okajima F Sphingosyl-phosphorylcholine Antagonizes Proton-Sensing Ovarian Cancer G-Protein-Coupled Receptor 1 (OGR1)-Mediated Inositol Phosphate Production and cAMP Accumulation. J. Pharmacol. Sci 2005, 99, 160–167. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases
Miscellaneous

