Studies Targeting Ryanodol Result in an Annulation Reaction for the Synthesis of a Variety of Fused Carbocycles
- PMID: 31298546
- PMCID: PMC6677624
- DOI: 10.1021/acs.orglett.9b02278
Studies Targeting Ryanodol Result in an Annulation Reaction for the Synthesis of a Variety of Fused Carbocycles
Abstract
An annulation reaction is described to access a range of polycyclic and highly oxygenated carbocycles. First developed in an approach to the synthesis of ryanodol, metallacycle-mediated annulative diketone-alkyne coupling defines a framework for realization of new retrosynthetic relationships for complex molecule synthesis. In addition to demonstrating this reaction in the context of forging distinct carbocyclic systems, including those featuring a seven-membered ring, the choice of quenching reagent leads to unique reaction outcomes.
Figures
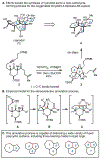
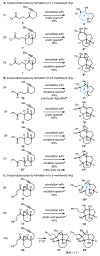
References
-
-
For recent reviews of natural product synthesis
- Nicolaou KC; Sorensen EJ; Wissinger N. The Art and Science of Organic and Natural Products Synthesis. J. Chem. Educ 1998, 75, 1225–1258.
- Nicolaou KC; Vourloumis D; Wissinger N; Baran PS The Art and Science of Total Synthesis at the Dawn of the Twenty-First Century. Angew. Chem. Int. Ed 2000, 39, 44–122. - PubMed
- Nicolaou KC; Hale CRH; Nilewskia C; Ioannidou HA Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance. Chem. Soc. Rev 2012, 41, 5185–5238. - PMC - PubMed
- Hoffmann RW Natural Product Synthesis: Changes over Time. Angew. Chem. Int. Ed 2013, 52, 123–130. - PubMed
- M. J Trying to rationalize total synthesis. Nat. Prod. Rep 2014, 31, 595–603. - PubMed
- Kuttruff CA; Eastgateb MD; Baran PS Natural product synthesis in the age of scalability. Nat. Prod. Rep 2014, 31, 419–432. - PubMed
- Zweig JE; Kim DE; Newhouse TR Methods Utilizing First-Row Transition Metals in Natural Product Total Synthesis. Chem. Rev 2017, 117, 11680–11752. - PubMed
- Hui C; Chen F; Pu F; Xu J Innovation in protecting-group-free natural product synthesis. Nat. Rev. Chem 2019, 3, 85–107.
-
-
- Bidasee KR; Besch HR Structure-Function Relationships among Ryanodine Derivatives. Pyridyl Ryanodine Definitively Separates Activation Potency from High Affinity. J. Biol. Chem 1998, 273, 12176–12186. - PubMed
- McPherson PS; Campbell KP The Ryanodine Receptor/Ca2+ Release Channel. J. Biol. Chem 1993, 268, 13765–13768. - PubMed
- Meissner G Ryanodine Receptor/Ca2+ Release Channels and Their Regulation by Endogenous Effectors. Annu. Rev. Physiol 1994, 56, 485–508. - PubMed
- Fill M; Copello JA Physiol. Rev 2002, 82, 893–922. - PubMed
- McCauley MD; Wehrens XHT Targeting Ryanodine Receptors for Anti-Arrhythmic Therapy. Acta Pharm. Sinic 2011, 32, 749–757. - PMC - PubMed
- Jenden DJ; Fairhurst AS The Pharmacology of Ryanodine. Pharmacol. Rev 1969, 21, 1–25. - PubMed
- Waterhouse AL; Pessah IN; Francini AO; Casida JE Structural Aspects of Ryanodine Action and Selectivity. J. Med. Chem 1987, 30, 710–716. - PubMed
- Wiesner K The Structure, Stereochemistry and Absolute Configuration of Anhydroryanodine. Pure Appl. Chem 1963, 7, 285–296.
- Wiesner K; Valenta Z; Findlay JA The Structure of Ryanodine. Tetrahedron Lett 1967, 8, 221–225.
- Srivastava SN; Przybylska M The Molecular Structure of Ryanodol-p-Bromo Benzyl Ether. Can. J. Chem 1968, 46, 795–797.
-
-
For examples of efforts targeting the total synthesis of ryanodol, see:
- Deslongchamps P. Synthetic Studies Towards Ryanodine. Pure Appl. Chem 1977, 49, 1329–1359.
- Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; MacLachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Saintlaurent L; Saintonge R; Soucy P; Ruest L; Deslongchamps P Total Synthesis of Ryanodol. Can. J. Chem 1979, 57, 3348–3354.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; MacLachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. Part I. General Strategy and Search for a Convenient Diene for the Construction of a Key Tricyclic Intermediate. Can. J. Chem 1990, 68, 115–126.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; MacLachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. Part II. Model Studies for Rings B and C of (+)-Anhydroryanodol. Preparation of a Key Pentacyclic Intermediate. Can. J. Chem 1990, 68, 127–152.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; MacLachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. Part III. Preparation of (+)-Anhydroryanodol from a Key Pentacyclic Intermediate. Can. J. Chem 1990, 68, 153–185.
- Deslongchamps P; Bélanger A; Berney DJF; Borschberg HJ; Brousseau R; Doutheau A; Durand R; Katayama H; Lapalme R; Leturc DM; Liao C-C; MacLachlan FN; Maffrand JP; Marazza F; Martino R; Moreau C; Ruest L; Saintlaurent L; Saintonge R; Soucy P The Total Synthesis of (+)-Ryanodol. Part IV. Preparation of (+)-Ryanodol from (+)-Anhydroryanodol. Can. J. Chem 1990, 68, 186–192.
- Nagatomo M; Koshimizu M; Masuda K; Tabuchi T; Urabe D; Inoue M Total Synthesis of Ryanodol. J. Am. Chem. Soc 2014, 136, 5916–5919. - PubMed
- Masuda K; Koshimizu M; Nagatomo M; Inoue M Asymmetric Total Synthesis of (+)-Ryanodol and (+)-Ryanodine. Chem. Eur. J 2016, 22, 230–236. - PubMed
- Chuang KV; Xu C; Reisman SE A 15-Step Synthesis of (+)-Ryanodol. Science, 2016, 353, 912–915. - PMC - PubMed
- Chen X; Han A; Virgil SC; Reisman SE Chemical Synthesis of (+)-Ryanodine and (+)-20-Deoxyspiganthine. ACS Cent. Sci 2017, 3, 278–282. - PMC - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

