A flexible design for advanced Phase I/II clinical trials with continuous efficacy endpoints
- PMID: 31298770
- PMCID: PMC6899762
- DOI: 10.1002/bimj.201800313
A flexible design for advanced Phase I/II clinical trials with continuous efficacy endpoints
Abstract
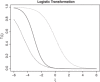
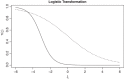
There is growing interest in integrated Phase I/II oncology clinical trials involving molecularly targeted agents (MTA). One of the main challenges of these trials are nontrivial dose-efficacy relationships and administration of MTAs in combination with other agents. While some designs were recently proposed for such Phase I/II trials, the majority of them consider the case of binary toxicity and efficacy endpoints only. At the same time, a continuous efficacy endpoint can carry more information about the agent's mechanism of action, but corresponding designs have received very limited attention in the literature. In this work, an extension of a recently developed information-theoretic design for the case of a continuous efficacy endpoint is proposed. The design transforms the continuous outcome using the logistic transformation and uses an information-theoretic argument to govern selection during the trial. The performance of the design is investigated in settings of single-agent and dual-agent trials. It is found that the novel design leads to substantial improvements in operating characteristics compared to a model-based alternative under scenarios with nonmonotonic dose/combination-efficacy relationships. The robustness of the design to missing/delayed efficacy responses and to the correlation in toxicity and efficacy endpoints is also investigated.
Keywords: Phase I/II clinical trial; combination trial; continuous endpoint; nonmonotonic efficacy.
© 2019 The Authors. Biometrical Journal Published by WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors have declared no conflict of interest.
Figures

Similar articles
-
An optimal Bayesian predictive probability design for phase II clinical trials with simple and complicated endpoints.Biom J. 2020 Mar;62(2):339-349. doi: 10.1002/bimj.201900022. Epub 2019 Aug 12. Biom J. 2020. PMID: 31402481
-
Bayesian dose-finding designs for combination of molecularly targeted agents assuming partial stochastic ordering.Stat Med. 2015 Feb 28;34(5):859-75. doi: 10.1002/sim.6376. Epub 2014 Nov 21. Stat Med. 2015. PMID: 25413162 Free PMC article.
-
UNITED: A Unified Transparent and Efficient Phase I/II Trial Design for Dose Optimization Accounting for Ordinal Graded, Continuous and Mixed Toxicity and Efficacy Endpoints.Stat Med. 2025 May;44(10-12):e70098. doi: 10.1002/sim.70098. Stat Med. 2025. PMID: 40384182 Free PMC article.
-
Determinants of the recommended phase 2 dose of molecular targeted agents.Cancer. 2017 Apr 15;123(8):1409-1415. doi: 10.1002/cncr.30579. Epub 2017 Feb 9. Cancer. 2017. PMID: 28182250
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
A seamless Phase I/II platform design with a time-to-event efficacy endpoint for potential COVID-19 therapies.Stat Methods Med Res. 2024 Nov;33(11-12):2115-2130. doi: 10.1177/09622802241288348. Epub 2024 Oct 14. Stat Methods Med Res. 2024. PMID: 39397762 Free PMC article.
-
Bayesian Safety and Futility Monitoring in Phase II Trials Using One Utility-Based Rule.Stat Med. 2024 Dec 20;43(29):5583-5595. doi: 10.1002/sim.10254. Epub 2024 Nov 5. Stat Med. 2024. PMID: 39497640 Free PMC article.
References
-
- Bekele, B. , & Shen, Y. (2005). A Bayesian approach to jointly modeling toxicity and biomarker expression in a Phase I/II dose‐finding trial. Biometrics, 61, 343–354. - PubMed
-
- Cheung, Y. K. (2005). Coherence principles in dose‐finding studies. Biometrika, 92, 863–873.
-
- Hirakawa, A. (2012). An adaptive dose‐finding approach for correlated bivariate binary and continuous outcomes in Phase I oncology trials. Statistics in Medicine, 31, 516–532. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

