Auxin-inducible protein degradation as a novel approach for protein depletion and reverse genetic discoveries in mammalian oocytes†
- PMID: 31299080
- PMCID: PMC6863966
- DOI: 10.1093/biolre/ioz113
Auxin-inducible protein degradation as a novel approach for protein depletion and reverse genetic discoveries in mammalian oocytes†
Abstract
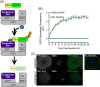
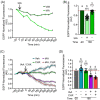
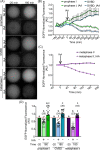
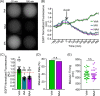
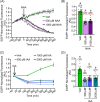
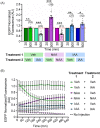
The disruption of protein expression is a major approach used for investigating protein function in mammalian oocytes. This is often achieved with RNAi/morpholino-mediated knockdown or gene knockout, leading to long-term loss of proteins of interest. However, these methods have noteworthy limitations, including (a) slow protein turnover can prohibit use of these approaches; (b) essential roles in early events precludes characterization of functions in subsequent events; (c) extended protein loss can allow time for compensatory mechanisms and other unanticipated events that confound interpretation of results. The work presented here examines the use of auxin-inducible degradation, a powerful new approach that overcomes these limitations through the depletion of one's protein of interest through controllable ubiquitin-mediated degradation. This method has been employed in yeast and mammalian cell lines, and here we demonstrate the utility of auxin-inducible degradation in mouse oocytes at multiple stages of meiosis, through degradation of exogenously expressed EGFP. We also evaluate important parameters for experimental design for use of this system in oocytes. This study thus expands the toolkit of researchers in oocyte biology, establishing the use of this unique and versatile approach for depleting proteins in oocytes, and providing researchers with valuable information to make use of this system.
Keywords: auxin-inducible degradation; meiosis; oocyte; protein depletion.
© The Author(s) 2019. Published by Oxford University Press on behalf of Society for the Study of Reproduction.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

