Ligand Identity-Induced Generation of Enhanced Oxidative Hydrogen Atom Transfer Reactivity for a CuII2(O2•-) Complex Driven by Formation of a CuII2(-OOH) Compound with a Strong O-H Bond
- PMID: 31299154
- PMCID: PMC6752211
- DOI: 10.1021/jacs.9b05277
Ligand Identity-Induced Generation of Enhanced Oxidative Hydrogen Atom Transfer Reactivity for a CuII2(O2•-) Complex Driven by Formation of a CuII2(-OOH) Compound with a Strong O-H Bond
Abstract
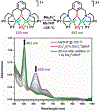
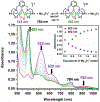
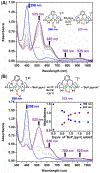
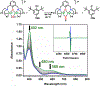
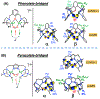
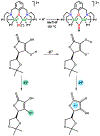
A superoxide-bridged dicopper(II) complex, [CuII2(XYLO)(O2•-)]2+ (1) (XYLO = binucleating m-xylyl derivative with a bridging phenolate ligand donor and two bis(2-{2-pyridyl}ethyl)amine arms), was generated from chemical oxidation of the peroxide-bridged dicopper(II) complex [CuII2(XYLO)(O22-)]+ (2), using ferrocenium (Fc+) derivatives, in 2-methyltetrahydrofuran (MeTHF) at -125 °C. Using Me10Fc+, a 1 ⇆ 2 equilibrium was established, allowing for calculation of the reduction potential of 1 as -0.525 ± 0.01 V vs Fc+/0. Addition of 1 equiv of strong acid to 2 afforded the hydroperoxide-bridged dicopper(II) species [CuII2(XYLO)(OOH)]2+ (3). An acid-base equilibrium between 3 and 2 was achieved through spectral titrations using a derivatized phosphazene base. The pKa of 3 was thus determined to be 24 ± 0.6 in MeTHF at -125 °C. Using a thermodynamic square scheme and the Bordwell relationship, the hydroperoxo complex (3) O-H bond dissociation free energy (BDFE) was calculated as 81.8 ± 1.5 (BDE = 86.8) kcal/mol. The observed oxidizing capability of [CuII2(XYLO)(O2•-)]2+ (1), as demonstrated in H atom abstraction reactions with certain phenolic ArO-H and hydrocarbon C-H substrates, provides direct support for this experimentally determined O-H BDFE. A kinetic study reveals a very fast reaction of TEMPO-H with 1 in MeTHF, with k (-100 °C) = 5.6 M-1 s-1. Density functional theory (DFT) calculations reveal how the structure of 1 may minimize stabilization of the superoxide moiety, resulting in its enhanced reactivity. The thermodynamic insights obtained herein highlight the importance of the interplay between ligand design and the generation and properties of copper (or other metal ion) bound O2-derived reduced species, such as pKa, reduction potential, and BDFE; these may be relevant to the capabilities (i.e., oxidizing power) of reactive oxygen intermediates in metalloenzyme chemical system mediated oxidative processes.
Figures




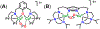
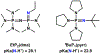




Similar articles
-
Heme-FeIII Superoxide, Peroxide and Hydroperoxide Thermodynamic Relationships: FeIII-O2•- Complex H-Atom Abstraction Reactivity.J Am Chem Soc. 2020 Feb 12;142(6):3104-3116. doi: 10.1021/jacs.9b12571. Epub 2020 Jan 28. J Am Chem Soc. 2020. PMID: 31913628 Free PMC article.
-
Peroxo and Superoxo Moieties Bound to Copper Ion: Electron-Transfer Equilibrium with a Small Reorganization Energy.J Am Chem Soc. 2016 Jun 8;138(22):7055-66. doi: 10.1021/jacs.6b02404. Epub 2016 May 26. J Am Chem Soc. 2016. PMID: 27228314 Free PMC article.
-
Coordination Variations within Binuclear Copper Dioxygen-Derived (Hydro)Peroxo and Superoxo Species; Influences upon Thermodynamic and Electronic Properties.J Am Chem Soc. 2024 May 15;146(19):13066-13082. doi: 10.1021/jacs.3c14422. Epub 2024 Apr 30. J Am Chem Soc. 2024. PMID: 38688016 Free PMC article.
-
[CuO](+) and [CuOH](2+) complexes: intermediates in oxidation catalysis?Acc Chem Res. 2015 Jul 21;48(7):2126-31. doi: 10.1021/acs.accounts.5b00169. Epub 2015 Jun 15. Acc Chem Res. 2015. PMID: 26075312 Free PMC article. Review.
-
Manganese-Oxygen Intermediates in O-O Bond Activation and Hydrogen-Atom Transfer Reactions.Acc Chem Res. 2017 Nov 21;50(11):2706-2717. doi: 10.1021/acs.accounts.7b00343. Epub 2017 Oct 24. Acc Chem Res. 2017. PMID: 29064667 Review.
Cited by
-
Copper-oxygen adducts: new trends in characterization and properties towards C-H activation.Chem Sci. 2024 May 13;15(27):10308-10349. doi: 10.1039/d4sc01762e. eCollection 2024 Jul 10. Chem Sci. 2024. PMID: 38994420 Free PMC article. Review.
-
Ferric Heme Superoxide Reductive Transformations to Ferric Heme (Hydro)Peroxide Species: Spectroscopic Characterization and Thermodynamic Implications for H-Atom Transfer (HAT).Angew Chem Int Ed Engl. 2021 Mar 8;60(11):5907-5912. doi: 10.1002/anie.202013791. Epub 2021 Feb 3. Angew Chem Int Ed Engl. 2021. PMID: 33348450 Free PMC article.
-
The rotamer of the second-sphere histidine in AA9 lytic polysaccharide monooxygenase is pH dependent.Biophys J. 2024 May 7;123(9):1139-1151. doi: 10.1016/j.bpj.2024.04.002. Epub 2024 Apr 2. Biophys J. 2024. PMID: 38571309 Free PMC article.
-
End-On Copper(I) Superoxo and Cu(II) Peroxo and Hydroperoxo Complexes Generated by Cryoreduction/Annealing and Characterized by EPR/ENDOR Spectroscopy.J Am Chem Soc. 2022 Jan 12;144(1):377-389. doi: 10.1021/jacs.1c10252. Epub 2022 Jan 4. J Am Chem Soc. 2022. PMID: 34981938 Free PMC article.
-
Synthetic Copper-(Di)oxygen Complex Generation and Reactivity Relevant to Copper Protein O2-Processing.Bull Jpn Soc Coord Chem. 2024;83:16-27. doi: 10.4019/bjscc.83.16. Epub 2024 Jun 20. Bull Jpn Soc Coord Chem. 2024. PMID: 39372915 Free PMC article.
References
-
- Pegis ML; Wise CF; Martin DJ; Mayer JM Oxygen Reduction by Homogeneous Molecular Catalysts and Electrocatalysts. Chem. Rev. 2018, 118, 2340–2391. - PubMed
-
- Mano N; de Poulpiquet A O2 Reduction in Enzymatic Biofuel Cells. Chem. Rev. 2018, 118, 2392–2468. - PubMed
-
- Mccann SD; Stahl SS Copper-Catalyzed Aerobic Oxidations of Organic Molecules: Pathways for Two-Electron Oxidation with a Four-Electron Oxidant and a One-Electron Redox-Active Catalyst. Acc. Chem. Res. 2015, 48, 1756–1766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

