mAKAPβ signalosomes - A nodal regulator of gene transcription associated with pathological cardiac remodeling
- PMID: 31299211
- PMCID: PMC7197268
- DOI: 10.1016/j.cellsig.2019.109357
mAKAPβ signalosomes - A nodal regulator of gene transcription associated with pathological cardiac remodeling
Abstract
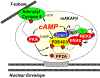
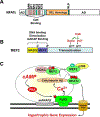
Striated myocytes compose about half of the cells of the heart, while contributing the majority of the heart's mass and volume. In response to increased demands for pumping power, including in diseases of pressure and volume overload, the contractile myocytes undergo non-mitotic growth, resulting in increased heart mass, i.e. cardiac hypertrophy. Myocyte hypertrophy is induced by a change in the gene expression program driven by the altered activity of transcription factors and co-repressor and co-activator chromatin-associated proteins. These gene regulatory proteins are subject to diverse post-translational modifications and serve as nuclear effectors for intracellular signal transduction pathways, including those controlled by cyclic nucleotides and calcium ion. Scaffold proteins contribute to the underlying architecture of intracellular signaling networks by targeting signaling enzymes to discrete intracellular compartments, providing specificity to the regulation of downstream effectors, including those regulating gene expression. Muscle A-kinase anchoring protein β (mAKAPβ) is a well-characterized scaffold protein that contributes to the regulation of pathological cardiac hypertrophy. In this review, we discuss the mechanisms how this prototypical scaffold protein organizes signalosomes responsible for the regulation of class IIa histone deacetylases and cardiac transcription factors such as NFAT, MEF2, and HIF-1α, as well as how this signalosome represents a novel therapeutic target for the prevention or treatment of heart failure.
Keywords: AKAP; Cardiac hypertrophy; Gene transcription; Kinase; cAMP.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

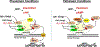

Similar articles
-
The scaffold protein muscle A-kinase anchoring protein β orchestrates cardiac myocyte hypertrophic signaling required for the development of heart failure.Circ Heart Fail. 2014 Jul;7(4):663-72. doi: 10.1161/CIRCHEARTFAILURE.114.001266. Epub 2014 May 8. Circ Heart Fail. 2014. PMID: 24812305 Free PMC article.
-
mAKAP-a master scaffold for cardiac remodeling.J Cardiovasc Pharmacol. 2015 Mar;65(3):218-25. doi: 10.1097/FJC.0000000000000206. J Cardiovasc Pharmacol. 2015. PMID: 25551320 Free PMC article. Review.
-
mAKAPβ signalosome: A potential target for cardiac hypertrophy.Drug Dev Res. 2023 Sep;84(6):1072-1084. doi: 10.1002/ddr.22081. Epub 2023 May 18. Drug Dev Res. 2023. PMID: 37203301 Review.
-
Signalosome-Regulated Serum Response Factor Phosphorylation Determining Myocyte Growth in Width Versus Length as a Therapeutic Target for Heart Failure.Circulation. 2020 Dec;142(22):2138-2154. doi: 10.1161/CIRCULATIONAHA.119.044805. Epub 2020 Sep 16. Circulation. 2020. PMID: 32933333 Free PMC article.
-
Bidirectional regulation of HDAC5 by mAKAPβ signalosomes in cardiac myocytes.J Mol Cell Cardiol. 2018 May;118:13-25. doi: 10.1016/j.yjmcc.2018.03.001. Epub 2018 Mar 6. J Mol Cell Cardiol. 2018. PMID: 29522762 Free PMC article.
Cited by
-
CaMKIIδC Drives Early Adaptive Ca2+ Change and Late Eccentric Cardiac Hypertrophy.Circ Res. 2020 Oct 9;127(9):1159-1178. doi: 10.1161/CIRCRESAHA.120.316947. Epub 2020 Aug 21. Circ Res. 2020. PMID: 32821022 Free PMC article.
-
The role of A-kinase anchoring proteins in cardiac oxidative stress.Biochem Soc Trans. 2019 Oct 31;47(5):1341-1353. doi: 10.1042/BST20190228. Biochem Soc Trans. 2019. PMID: 31671182 Free PMC article. Review.
-
Reversal of injury-associated retinal ganglion cell gene expression by a phosphodiesterase anchoring disruptor peptide.Exp Eye Res. 2024 Sep;246:110017. doi: 10.1016/j.exer.2024.110017. Epub 2024 Aug 2. Exp Eye Res. 2024. PMID: 39097072
-
Regulation of cardiac function by cAMP nanodomains.Biosci Rep. 2023 Feb 27;43(2):BSR20220953. doi: 10.1042/BSR20220953. Biosci Rep. 2023. PMID: 36749130 Free PMC article. Review.
-
Calcineurin in the heart: New horizons for an old friend.Cell Signal. 2021 Nov;87:110134. doi: 10.1016/j.cellsig.2021.110134. Epub 2021 Aug 25. Cell Signal. 2021. PMID: 34454008 Free PMC article. Review.
References
-
- Benjamin EJ, et al., Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation, 2018. 137(12): p. e67–e492. - PubMed
-
- Benjamin EJ, et al., Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

