Aging negatively impacts the ability of megakaryocytes to stimulate osteoblast proliferation and bone mass
- PMID: 31299382
- PMCID: PMC6708771
- DOI: 10.1016/j.bone.2019.07.010
Aging negatively impacts the ability of megakaryocytes to stimulate osteoblast proliferation and bone mass
Abstract
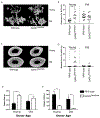
Osteoblast number and activity decreases with aging, contributing to the age-associated decline of bone mass, but the mechanisms underlying changes in osteoblast activity are not well understood. Here, we show that the age-associated bone loss critically depends on impairment of the ability of megakaryocytes (MKs) to support osteoblast proliferation. Co-culture of osteoblast precursors with young MKs is known to increase osteoblast proliferation and bone formation. However, co-culture of osteoblast precursors with aged MKs resulted in significantly fewer osteoblasts compared to co-culture with young MKs, and this was associated with the downregulation of transforming growth factor beta. In addition, the ability of MKs to increase bone mass was attenuated during aging as transplantation of GATA1low/low hematopoietic donor cells (which have elevated MKs/MK precursors) from young mice resulted in an increase in bone mass of recipient mice compared to transplantation of young wild-type donor cells, whereas transplantation of GATA1low/low donor cells from old mice failed to enhance bone mass in recipient mice compared to transplantation of old wild-type donor cells. These findings suggest that the preservation or restoration of the MK-mediated induction of osteoblast proliferation during aging may hold the potential to prevent age-associated bone loss and resulting fractures.
Keywords: Aging; Bone loss; Bone mass; Megakaryocytes; Osteoblasts.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Immature and mature megakaryocytes enhance osteoblast proliferation and inhibit osteoclast formation.J Cell Biochem. 2010 Mar 1;109(4):774-81. doi: 10.1002/jcb.22456. J Cell Biochem. 2010. PMID: 20052670 Free PMC article.
-
Pyk2 and Megakaryocytes Regulate Osteoblast Differentiation and Migration Via Distinct and Overlapping Mechanisms.J Cell Biochem. 2016 Jun;117(6):1396-406. doi: 10.1002/jcb.25430. Epub 2015 Dec 10. J Cell Biochem. 2016. PMID: 26552846 Free PMC article.
-
Megakaryocytes are mechanically responsive and influence osteoblast proliferation and differentiation.Bone. 2014 Sep;66:111-20. doi: 10.1016/j.bone.2014.05.015. Epub 2014 Jun 2. Bone. 2014. PMID: 24882736 Free PMC article.
-
A reciprocal regulatory interaction between megakaryocytes, bone cells, and hematopoietic stem cells.Bone. 2006 Nov;39(5):978-984. doi: 10.1016/j.bone.2006.05.019. Epub 2006 Jul 21. Bone. 2006. PMID: 16860008 Review.
-
Megakaryocyte-bone cell interactions.Adv Exp Med Biol. 2010;658:31-41. doi: 10.1007/978-1-4419-1050-9_4. Adv Exp Med Biol. 2010. PMID: 19950013 Review.
Cited by
-
IL-33/IL-31 Axis in Osteoporosis.Int J Mol Sci. 2020 Feb 13;21(4):1239. doi: 10.3390/ijms21041239. Int J Mol Sci. 2020. PMID: 32069819 Free PMC article. Review.
-
STING signaling in inflammaging: a new target against musculoskeletal diseases.Front Immunol. 2023 Jul 10;14:1227364. doi: 10.3389/fimmu.2023.1227364. eCollection 2023. Front Immunol. 2023. PMID: 37492580 Free PMC article. Review.
-
Analysis of the effects of spaceflight and local administration of thrombopoietin to a femoral defect injury on distal skeletal sites.NPJ Microgravity. 2021 Mar 26;7(1):12. doi: 10.1038/s41526-021-00140-0. NPJ Microgravity. 2021. PMID: 33772025 Free PMC article.
-
15-PGDH inhibition activates the splenic niche to promote hematopoietic regeneration.JCI Insight. 2021 Mar 22;6(6):e143658. doi: 10.1172/jci.insight.143658. JCI Insight. 2021. PMID: 33600377 Free PMC article.
-
Roadmap for alleviating the manifestations of ageing in the cardiovascular system.Nat Rev Cardiol. 2025 Aug;22(8):577-605. doi: 10.1038/s41569-025-01130-5. Epub 2025 Feb 19. Nat Rev Cardiol. 2025. PMID: 39972009 Review.
References
-
- Hattner R, Epker BN, and Frost HM, Suggested sequential mode of control of changes In cell behaviour in adult bone remodelling. Nature, 1965. 206(983): p. 489–90. - PubMed
-
- Henriksen K, Karsdal MA, and Martin TJ, Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int, 2014. 94(1): p. 88–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

